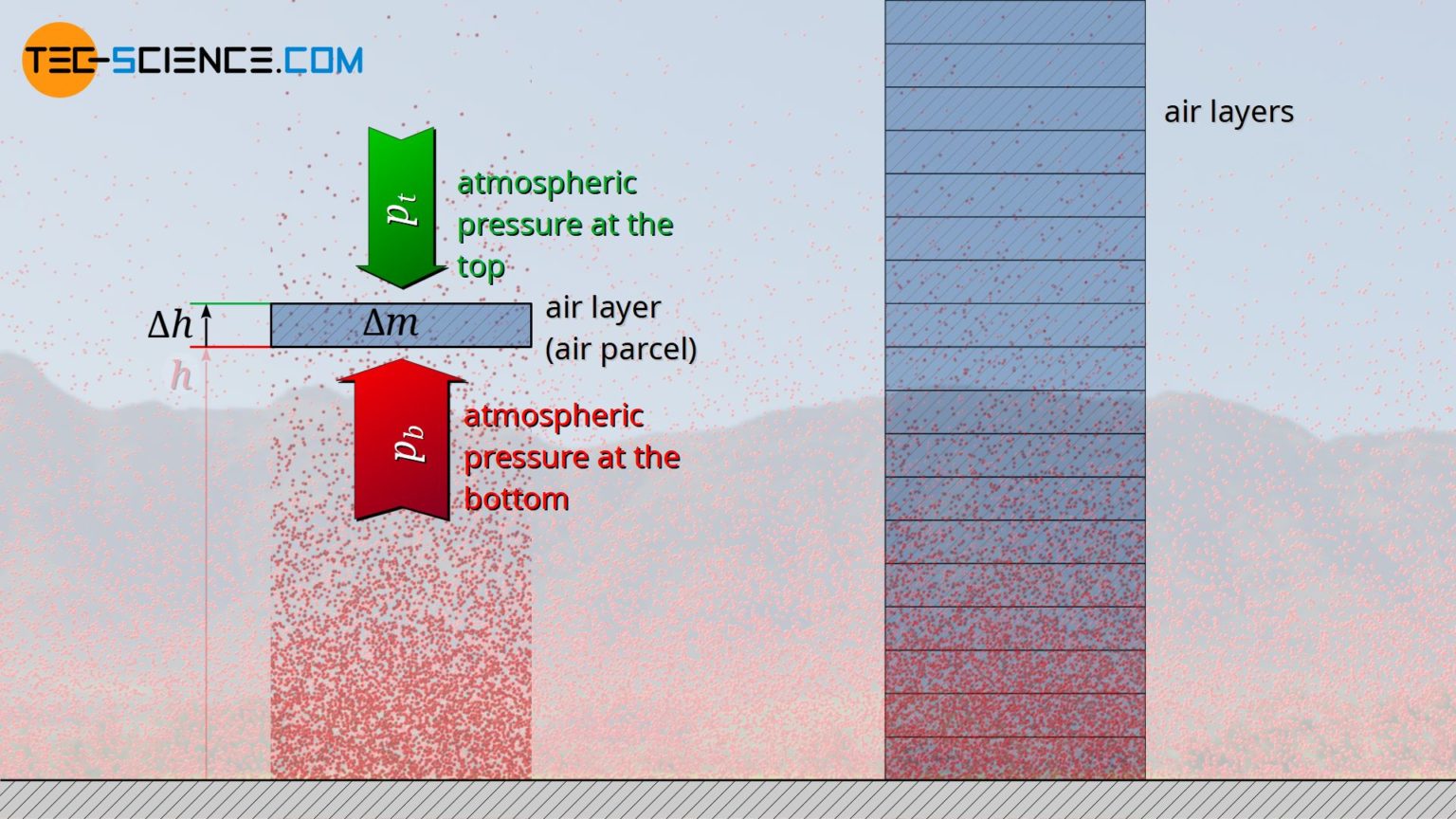
Barometric Formula Derivation . If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that the barometric formula can be expressed in terms of these number densities as The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure. It is convenient to define a scale height h for the atmosphere: Either of the latter relationships is frequently called the barometric formula. (2.10) leading to a compact form of the barometric law: The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. Equation (2.9) is called the barometric law. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0!
from www.tec-science.com
Either of the latter relationships is frequently called the barometric formula. The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that the barometric formula can be expressed in terms of these number densities as Equation (2.9) is called the barometric law. It is convenient to define a scale height h for the atmosphere: (2.10) leading to a compact form of the barometric law:
Derivation of the barometric formula (isothermal atmosphere) tecscience
Barometric Formula Derivation The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! Either of the latter relationships is frequently called the barometric formula. It is convenient to define a scale height h for the atmosphere: If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that the barometric formula can be expressed in terms of these number densities as Equation (2.9) is called the barometric law. The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure. (2.10) leading to a compact form of the barometric law: The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated.
From www.slideserve.com
PPT Review of the Gas Laws PowerPoint Presentation, free download Barometric Formula Derivation It is convenient to define a scale height h for the atmosphere: (2.10) leading to a compact form of the barometric law: The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! Either of the latter relationships is frequently called the barometric formula. The barometric formula. Barometric Formula Derivation.
From www.slideserve.com
PPT Lecture 5 Barometric formula and the Boltzmann equation Barometric Formula Derivation The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. The barometric formula for an adiabatic atmosphere takes into account the. Barometric Formula Derivation.
From www.studypool.com
SOLUTION Derivation of Barometric Equation Studypool Barometric Formula Derivation Either of the latter relationships is frequently called the barometric formula. The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. (2.10) leading to a compact form of the barometric law: It is convenient to define a scale height h for the atmosphere: The barometric. Barometric Formula Derivation.
From www.tec-science.com
Derivation of the barometric formula (isothermal atmosphere) tecscience Barometric Formula Derivation The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure. If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that the barometric formula can be expressed in terms of these number densities. Barometric Formula Derivation.
From dxoxcjdmd.blob.core.windows.net
Typical Barometric Pressure Variation at Britney Sloan blog Barometric Formula Derivation If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that the barometric formula can be expressed in terms of these number densities as The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure. Barometric Formula Derivation.
From www.tec-science.com
Derivation of the barometric formula (isothermal atmosphere) tecscience Barometric Formula Derivation It is convenient to define a scale height h for the atmosphere: Equation (2.9) is called the barometric law. Either of the latter relationships is frequently called the barometric formula. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The barometric formula for an adiabatic. Barometric Formula Derivation.
From www.tec-science.com
Derivation of the barometric formula (adiabatic atmosphere) tecscience Barometric Formula Derivation The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. Either of the latter relationships is frequently called the barometric formula. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0!. Barometric Formula Derivation.
From www.slideserve.com
PPT Lecture 5 Barometric formula and the Boltzmann equation Barometric Formula Derivation It is convenient to define a scale height h for the atmosphere: The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure. Barometric Formula Derivation.
From www.tec-science.com
Derivation of the barometric formula (isothermal atmosphere) tecscience Barometric Formula Derivation Equation (2.9) is called the barometric law. The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure. It is convenient to define a scale height h for the atmosphere: Either of the latter relationships is frequently called the barometric formula. If we let \(\eta\) be the. Barometric Formula Derivation.
From loejgfpbx.blob.core.windows.net
Barometric Equation at Hugh Clark blog Barometric Formula Derivation It is convenient to define a scale height h for the atmosphere: Equation (2.9) is called the barometric law. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write. Barometric Formula Derivation.
From www.youtube.com
Derivation of Barometric Formula Barometric Distribution LawUrdu Barometric Formula Derivation Equation (2.9) is called the barometric law. (2.10) leading to a compact form of the barometric law: The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure. The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the. Barometric Formula Derivation.
From www.tec-science.com
Derivation of the barometric formula (adiabatic atmosphere) tecscience Barometric Formula Derivation If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that the barometric formula can be expressed in terms of these number densities as (2.10) leading to a compact form of the barometric law: Either of the latter relationships is frequently called the barometric formula. It is. Barometric Formula Derivation.
From en.ppt-online.org
Physics 1 for KMA online presentation Barometric Formula Derivation Equation (2.9) is called the barometric law. The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. It is convenient to define a scale height h for the atmosphere: (2.10) leading to a compact form of the barometric law: The barometric formula for an adiabatic. Barometric Formula Derivation.
From byjus.com
Explain the how the variation of atmospheric pressure with height is Barometric Formula Derivation The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure. Equation (2.9) is called the barometric law. It is convenient to define a scale height h for the atmosphere: If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write. Barometric Formula Derivation.
From www.slideserve.com
PPT Review of the Gas Laws PowerPoint Presentation, free download Barometric Formula Derivation If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that the barometric formula can be expressed in terms of these number densities as The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and. Barometric Formula Derivation.
From www.youtube.com
How to calculate barometric altitude YouTube Barometric Formula Derivation The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure. The barometric formula, relating the pressure p(z) of an isothermal, ideal. Barometric Formula Derivation.
From www.scribd.com
Barometric Formula Wikipedia PDF Transparent Materials Barometric Formula Derivation (2.10) leading to a compact form of the barometric law: Equation (2.9) is called the barometric law. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! Either of the latter relationships is frequently called the barometric formula. The barometric formula for an adiabatic atmosphere takes. Barometric Formula Derivation.
From www.tec-science.com
Derivation of the barometric formula (isothermal atmosphere) tecscience Barometric Formula Derivation Equation (2.9) is called the barometric law. (2.10) leading to a compact form of the barometric law: If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that the barometric formula can be expressed in terms of these number densities as The development of the barometric formula. Barometric Formula Derivation.
From www.tec-science.com
Derivation of the barometric formula (adiabatic atmosphere) tecscience Barometric Formula Derivation The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure. It is convenient to define a scale height h for the atmosphere: (2.10) leading to a compact form of the barometric law: The development of the barometric formula makes use of a number of concepts from. Barometric Formula Derivation.
From www.youtube.com
Barometric formula(lecture 1)Professor Aziz Atif YouTube Barometric Formula Derivation The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! (2.10) leading to a compact form of the barometric law: If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that the barometric. Barometric Formula Derivation.
From www.youtube.com
Barometric Equation Derivation. YouTube Barometric Formula Derivation (2.10) leading to a compact form of the barometric law: It is convenient to define a scale height h for the atmosphere: The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. Equation (2.9) is called the barometric law. The barometric formula for an adiabatic. Barometric Formula Derivation.
From www.tec-science.com
Derivation of the barometric formula (isothermal atmosphere) tecscience Barometric Formula Derivation Equation (2.9) is called the barometric law. The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! Either of the latter. Barometric Formula Derivation.
From www.studypool.com
SOLUTION Derivation of Barometric Equation Studypool Barometric Formula Derivation Either of the latter relationships is frequently called the barometric formula. The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure. If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that the. Barometric Formula Derivation.
From loejgfpbx.blob.core.windows.net
Barometric Equation at Hugh Clark blog Barometric Formula Derivation The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that the barometric formula can be expressed in terms of these number. Barometric Formula Derivation.
From www.slideserve.com
PPT Lecture 5 Barometric formula and the Boltzmann equation Barometric Formula Derivation The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure. Either of the latter relationships is frequently called the barometric formula.. Barometric Formula Derivation.
From www.tec-science.com
Derivation of the barometric formula (isothermal atmosphere) tecscience Barometric Formula Derivation (2.10) leading to a compact form of the barometric law: The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! Equation. Barometric Formula Derivation.
From dxoqelgkm.blob.core.windows.net
Barometric Pressure Equation at Tricia Kearse blog Barometric Formula Derivation The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. Equation (2.9) is called the barometric law. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! (2.10) leading to a. Barometric Formula Derivation.
From www.studypool.com
SOLUTION Derivation of Barometric Equation Studypool Barometric Formula Derivation The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! It is convenient to define a scale height h for the atmosphere: If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that. Barometric Formula Derivation.
From www.slideserve.com
PPT Lecture 5 Barometric formula and the Boltzmann equation Barometric Formula Derivation It is convenient to define a scale height h for the atmosphere: The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure.. Barometric Formula Derivation.
From www.youtube.com
Barometric Formula Part 2 YouTube Barometric Formula Derivation If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that the barometric formula can be expressed in terms of these number densities as The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and. Barometric Formula Derivation.
From slidetodoc.com
Lecture 4 Barometric formula and the Boltzmann equation Barometric Formula Derivation If we let \(\eta\) be the number of molecules per unit volume, \(\eta ={n}/{v}\), we can write \(p={nkt}/{v}=\eta kt\) and \(p_0={\eta }_0kt\) so that the barometric formula can be expressed in terms of these number densities as The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure. Barometric Formula Derivation.
From www.studypool.com
SOLUTION Derivation of Barometric Equation Studypool Barometric Formula Derivation (2.10) leading to a compact form of the barometric law: Either of the latter relationships is frequently called the barometric formula. Equation (2.9) is called the barometric law. The development of the barometric formula makes use of a number of concepts from kinetic theory, such as the ideal gas law and the associated. It is convenient to define a scale. Barometric Formula Derivation.
From www.slideserve.com
PPT Review of the Gas Laws PowerPoint Presentation, free download Barometric Formula Derivation The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure. (2.10) leading to a compact form of the barometric law: Equation (2.9) is called the barometric law. The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height. Barometric Formula Derivation.
From www.tec-science.com
Derivation of the barometric formula (adiabatic atmosphere) tecscience Barometric Formula Derivation Equation (2.9) is called the barometric law. (2.10) leading to a compact form of the barometric law: The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the. Barometric Formula Derivation.
From www.tec-science.com
Derivation of the barometric formula (adiabatic atmosphere) tecscience Barometric Formula Derivation It is convenient to define a scale height h for the atmosphere: The barometric formula, relating the pressure p(z) of an isothermal, ideal gas of molecular mass m at some height z to its pressure p~0! The barometric formula for an adiabatic atmosphere takes into account the decrease in temperature with increasing altitude and the associated effects on air pressure.. Barometric Formula Derivation.