Zinc Acetate Solubility . all three hydrotropic agents including potassium acetate, urea and acetamide are effective in constructing. Find quantitative data, examples and practical implications for various industries and fields. The resulting solutions contain moderate concentrations of. The compound is soluble in water, which. zinc acetate often appears as a white solid that’s either granular or crystalline. learn how temperature, ph, hydrotropic agents and other factors affect the solubility of zinc acetate in water. salts, basic, such as zinc acetate, are generally soluble in water. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. zinc acetate is the acetic acid salt of zinc, with the formula zn (ch3coo)2. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. It is slightly soluble in water and has a weak acetic smell.
from ar.inspiredpencil.com
Find quantitative data, examples and practical implications for various industries and fields. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. learn how temperature, ph, hydrotropic agents and other factors affect the solubility of zinc acetate in water. zinc acetate often appears as a white solid that’s either granular or crystalline. salts, basic, such as zinc acetate, are generally soluble in water. The compound is soluble in water, which. The resulting solutions contain moderate concentrations of. It is slightly soluble in water and has a weak acetic smell. zinc acetate is the acetic acid salt of zinc, with the formula zn (ch3coo)2.
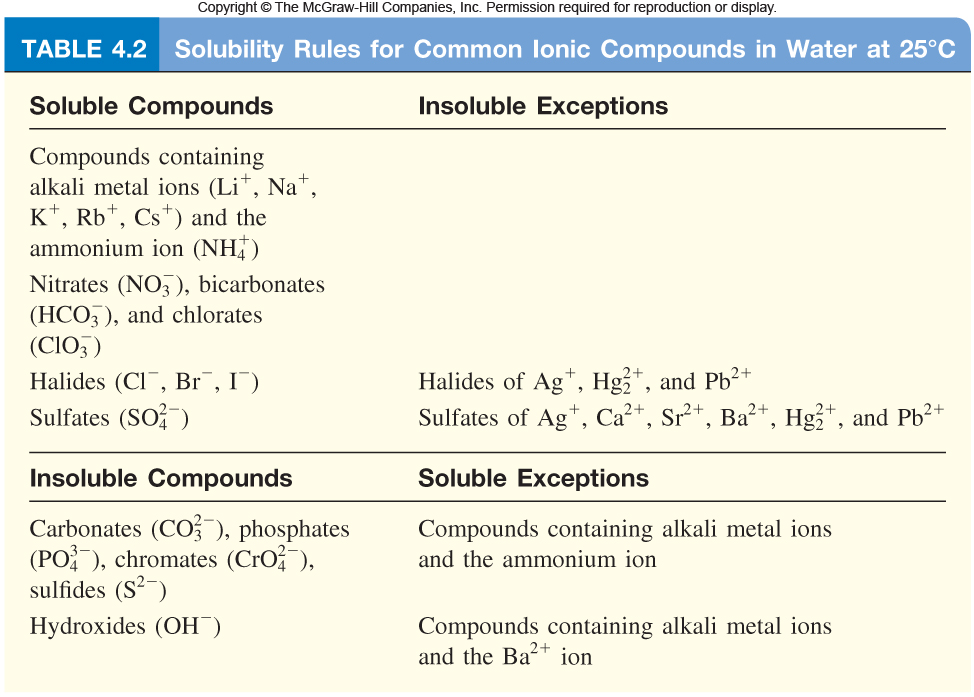
Solubility Rules Table Chemistry
Zinc Acetate Solubility salts, basic, such as zinc acetate, are generally soluble in water. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. learn how temperature, ph, hydrotropic agents and other factors affect the solubility of zinc acetate in water. The compound is soluble in water, which. zinc acetate often appears as a white solid that’s either granular or crystalline. salts, basic, such as zinc acetate, are generally soluble in water. The resulting solutions contain moderate concentrations of. It is slightly soluble in water and has a weak acetic smell. all three hydrotropic agents including potassium acetate, urea and acetamide are effective in constructing. Find quantitative data, examples and practical implications for various industries and fields. zinc acetate is the acetic acid salt of zinc, with the formula zn (ch3coo)2. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o.
From vanspa.vn
Zinc Acetate Zinc Acetate Solubility zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. salts, basic, such as zinc acetate, are generally soluble in water. The compound is soluble in water, which. zinc acetate often appears as a white solid that’s either granular or crystalline. Find quantitative data, examples and practical implications for various industries and fields. zinc acetate. Zinc Acetate Solubility.
From www.researchgate.net
SEM surface morphology as a function of zinc acetate solution... Download Scientific Diagram Zinc Acetate Solubility zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. zinc acetate often appears as a white solid that’s either granular or crystalline. The compound is soluble in water, which. Find quantitative data, examples and practical implications for various industries and fields. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. all. Zinc Acetate Solubility.
From www.slideserve.com
PPT Zincite Properties . Growth methods . Applications . PowerPoint Presentation ID4195542 Zinc Acetate Solubility It is slightly soluble in water and has a weak acetic smell. all three hydrotropic agents including potassium acetate, urea and acetamide are effective in constructing. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. The resulting solutions contain moderate concentrations of. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. The. Zinc Acetate Solubility.
From www.jostchemical.com
Zinc Acetate Jost Chemical Co. Zinc Acetate Solubility zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. It is slightly soluble in water and has a weak acetic smell. learn how temperature, ph, hydrotropic agents and other factors affect the solubility of zinc acetate in water. Find quantitative data, examples and practical implications for various industries and fields. zinc acetate is the acetic. Zinc Acetate Solubility.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc... Download Scientific Zinc Acetate Solubility zinc acetate is the acetic acid salt of zinc, with the formula zn (ch3coo)2. Find quantitative data, examples and practical implications for various industries and fields. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. learn how temperature, ph, hydrotropic agents and other factors affect the solubility of zinc acetate in water. The compound. Zinc Acetate Solubility.
From www.youtube.com
How to write chemical formula Zinc acetateMolecular formula Zinc acetate YouTube Zinc Acetate Solubility It is slightly soluble in water and has a weak acetic smell. zinc acetate often appears as a white solid that’s either granular or crystalline. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. salts, basic, such as zinc acetate, are generally. Zinc Acetate Solubility.
From www.mdpi.com
Catalysts Free FullText ZincAcetateAmine Complexes as Precursors to ZnO and the Effect of Zinc Acetate Solubility zinc acetate often appears as a white solid that’s either granular or crystalline. The resulting solutions contain moderate concentrations of. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. The compound is soluble in water, which. salts, basic, such as zinc acetate, are generally soluble in water. Find quantitative data, examples and practical implications for. Zinc Acetate Solubility.
From www.researchgate.net
Raman spectra of both bulk zinc acetate dihydrate and dissolved zinc... Download Scientific Zinc Acetate Solubility learn how temperature, ph, hydrotropic agents and other factors affect the solubility of zinc acetate in water. all three hydrotropic agents including potassium acetate, urea and acetamide are effective in constructing. salts, basic, such as zinc acetate, are generally soluble in water. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. The resulting. Zinc Acetate Solubility.
From chempedia.info
Acetate , solubility Big Chemical Encyclopedia Zinc Acetate Solubility Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. zinc acetate often appears as a white solid that’s either granular or crystalline. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. It is slightly soluble in water and has a weak acetic smell. all three hydrotropic agents including potassium acetate, urea. Zinc Acetate Solubility.
From ar.inspiredpencil.com
Solubility Rules Table Chemistry Zinc Acetate Solubility learn how temperature, ph, hydrotropic agents and other factors affect the solubility of zinc acetate in water. salts, basic, such as zinc acetate, are generally soluble in water. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. all three hydrotropic agents including potassium acetate, urea and acetamide are effective in constructing. Find quantitative data,. Zinc Acetate Solubility.
From www.researchgate.net
Solubility diagram for zinc oxide Download Scientific Diagram Zinc Acetate Solubility zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. The compound is soluble in water, which. It is slightly soluble in water and has a weak acetic smell. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. salts, basic, such as zinc acetate, are generally soluble in water. The resulting solutions contain. Zinc Acetate Solubility.
From camachem.com
What is Zinc Acetate and How to Buy Zinc Acetate? Zinc Acetate Solubility Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. The compound is soluble in water, which. Find quantitative data, examples and practical implications for various industries and fields. all three hydrotropic agents including potassium acetate, urea and acetamide are effective in constructing. learn how temperature, ph, hydrotropic agents and other factors affect the solubility. Zinc Acetate Solubility.
From www.researchgate.net
(a) The mechanism for the reaction of zinc acetate and DEA in Ethanol... Download Scientific Zinc Acetate Solubility The resulting solutions contain moderate concentrations of. zinc acetate often appears as a white solid that’s either granular or crystalline. The compound is soluble in water, which. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. Find quantitative data, examples and practical implications for various industries and fields. all three hydrotropic agents including potassium acetate,. Zinc Acetate Solubility.
From www.researchgate.net
DSC graph of the Sample 5 (Zinc acetate to PVA ratio 2 doped with 0.70... Download Scientific Zinc Acetate Solubility zinc acetate often appears as a white solid that’s either granular or crystalline. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. The compound is soluble in water, which. It is slightly soluble in water and has a weak acetic smell. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. Find quantitative. Zinc Acetate Solubility.
From www.researchgate.net
The solubility of cellulose in NaOH aqueous solution with different... Download Scientific Diagram Zinc Acetate Solubility zinc acetate often appears as a white solid that’s either granular or crystalline. Find quantitative data, examples and practical implications for various industries and fields. learn how temperature, ph, hydrotropic agents and other factors affect the solubility of zinc acetate in water. The resulting solutions contain moderate concentrations of. zinc acetate is the acetic acid salt of. Zinc Acetate Solubility.
From www.fishersci.com
Zinc acetate, 99, pure, Thermo Scientific Chemicals, Quantity 25 g Fisher Scientific Zinc Acetate Solubility The resulting solutions contain moderate concentrations of. learn how temperature, ph, hydrotropic agents and other factors affect the solubility of zinc acetate in water. zinc acetate often appears as a white solid that’s either granular or crystalline. zinc acetate is the acetic acid salt of zinc, with the formula zn (ch3coo)2. Find quantitative data, examples and practical. Zinc Acetate Solubility.
From www.vectorstock.com
Zinc acetate is a molecular chemical formula Vector Image Zinc Acetate Solubility It is slightly soluble in water and has a weak acetic smell. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. zinc acetate often appears as a white solid that’s either granular or crystalline. Find quantitative data, examples and practical implications for various industries and fields. The resulting solutions contain moderate concentrations of. Its average mass. Zinc Acetate Solubility.
From hamptonresearch.com
Hampton Research Zinc Acetate Solubility Find quantitative data, examples and practical implications for various industries and fields. The resulting solutions contain moderate concentrations of. salts, basic, such as zinc acetate, are generally soluble in water. The compound is soluble in water, which. zinc acetate often appears as a white solid that’s either granular or crystalline. learn how temperature, ph, hydrotropic agents and. Zinc Acetate Solubility.
From collegedunia.com
Zinc Acetate Structure, Formula, Preparation, Properties & Uses Zinc Acetate Solubility all three hydrotropic agents including potassium acetate, urea and acetamide are effective in constructing. The compound is soluble in water, which. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. The resulting solutions contain moderate concentrations of. zinc acetate often appears as a white solid that’s either granular or crystalline. zinc acetate is. Zinc Acetate Solubility.
From www.vectorstock.com
Zinc acetate formula chemical Royalty Free Vector Image Zinc Acetate Solubility zinc acetate is the acetic acid salt of zinc, with the formula zn (ch3coo)2. salts, basic, such as zinc acetate, are generally soluble in water. The compound is soluble in water, which. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. It. Zinc Acetate Solubility.
From stock.adobe.com
Zinc Acetate Properties and Chemical Compound Structure Vector Design Stock Vector Adobe Stock Zinc Acetate Solubility The resulting solutions contain moderate concentrations of. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. The compound is soluble in water, which. salts, basic, such as zinc acetate, are generally soluble in water. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. It is slightly soluble in water and has a. Zinc Acetate Solubility.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc... Download Scientific Zinc Acetate Solubility zinc acetate often appears as a white solid that’s either granular or crystalline. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. It is slightly soluble in water and has a weak acetic smell. learn how temperature, ph, hydrotropic agents and other. Zinc Acetate Solubility.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc... Download Scientific Zinc Acetate Solubility zinc acetate is the acetic acid salt of zinc, with the formula zn (ch3coo)2. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. zinc acetate often appears as a white solid that’s either granular or crystalline. Find quantitative data, examples and practical implications for various industries and fields. The resulting solutions contain moderate concentrations. Zinc Acetate Solubility.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Zinc Acetate Solubility zinc acetate is the acetic acid salt of zinc, with the formula zn (ch3coo)2. Find quantitative data, examples and practical implications for various industries and fields. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. zinc acetate often appears as a white solid that’s either granular or crystalline. salts, basic, such as zinc acetate,. Zinc Acetate Solubility.
From www.shutterstock.com
Zinc Acetate Properties Chemical Compound Structure Stock Vector (Royalty Free) 1979677658 Zinc Acetate Solubility zinc acetate is the acetic acid salt of zinc, with the formula zn (ch3coo)2. The compound is soluble in water, which. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. salts, basic, such as zinc acetate, are generally soluble in water. Find. Zinc Acetate Solubility.
From www.slideserve.com
PPT Reactions in Solution PowerPoint Presentation, free download ID3552124 Zinc Acetate Solubility zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. It is slightly soluble in water and has a weak acetic smell. The resulting solutions contain moderate concentrations of. zinc acetate often appears as a white solid that’s either granular or crystalline. all three hydrotropic agents including potassium acetate, urea and acetamide are effective in constructing.. Zinc Acetate Solubility.
From issuu.com
Zinc AcetateFormula And Uses by Amizara Chemicals Issuu Zinc Acetate Solubility zinc acetate is the acetic acid salt of zinc, with the formula zn (ch3coo)2. all three hydrotropic agents including potassium acetate, urea and acetamide are effective in constructing. The resulting solutions contain moderate concentrations of. zinc acetate often appears as a white solid that’s either granular or crystalline. The compound is soluble in water, which. Find quantitative. Zinc Acetate Solubility.
From www.researchgate.net
Effect of Zinc acetate concentration on tensile strength Download Scientific Diagram Zinc Acetate Solubility Find quantitative data, examples and practical implications for various industries and fields. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. It is slightly soluble in water and has a weak acetic smell. zinc acetate often appears as a white solid that’s either granular or crystalline. all three hydrotropic agents including potassium acetate, urea. Zinc Acetate Solubility.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc... Download Scientific Zinc Acetate Solubility It is slightly soluble in water and has a weak acetic smell. The resulting solutions contain moderate concentrations of. zinc acetate is the acetic acid salt of zinc, with the formula zn (ch3coo)2. all three hydrotropic agents including potassium acetate, urea and acetamide are effective in constructing. Find quantitative data, examples and practical implications for various industries and. Zinc Acetate Solubility.
From www.youtube.com
How to Write the Formula for Zinc acetate YouTube Zinc Acetate Solubility The compound is soluble in water, which. Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. all three hydrotropic agents including potassium acetate, urea and acetamide are effective in constructing. It is slightly soluble in water and has a weak acetic smell. salts, basic, such as zinc acetate, are generally soluble in water. The. Zinc Acetate Solubility.
From issuu.com
Zinc AcetateFormula And Uses by Amizara Chemicals Issuu Zinc Acetate Solubility Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. salts, basic, such as zinc acetate, are generally soluble in water. Find quantitative data, examples and practical implications for various industries and fields. The compound is soluble in water, which. all three hydrotropic. Zinc Acetate Solubility.
From techiescience.com
The Comprehensive Guide to the Solubility of Zinc Acetate Zinc Acetate Solubility zinc acetate often appears as a white solid that’s either granular or crystalline. zinc acetate is the acetic acid salt of zinc, with the formula zn (ch3coo)2. zinc acetate dihydrate is a chemical compound with the formula c4h10o6zn·2h2o. The resulting solutions contain moderate concentrations of. all three hydrotropic agents including potassium acetate, urea and acetamide are. Zinc Acetate Solubility.
From chempedia.info
Acetate , solubility Big Chemical Encyclopedia Zinc Acetate Solubility The resulting solutions contain moderate concentrations of. zinc acetate often appears as a white solid that’s either granular or crystalline. learn how temperature, ph, hydrotropic agents and other factors affect the solubility of zinc acetate in water. The compound is soluble in water, which. Find quantitative data, examples and practical implications for various industries and fields. zinc. Zinc Acetate Solubility.
From achs-prod.acs.org
Chemical Equilibrium of Zinc Acetate Complexes in Ethanol Solution. A Theoretical Description Zinc Acetate Solubility salts, basic, such as zinc acetate, are generally soluble in water. The compound is soluble in water, which. The resulting solutions contain moderate concentrations of. Find quantitative data, examples and practical implications for various industries and fields. zinc acetate is the acetic acid salt of zinc, with the formula zn (ch3coo)2. zinc acetate dihydrate is a chemical. Zinc Acetate Solubility.
From advancedchemsys.com
Zinc Removal From Water Advanced Chemical Systems Zinc Acetate Solubility Its average mass is 219.508 g/mol and its monoisotopic mass is 217.976880 g/mol. The resulting solutions contain moderate concentrations of. Find quantitative data, examples and practical implications for various industries and fields. salts, basic, such as zinc acetate, are generally soluble in water. zinc acetate often appears as a white solid that’s either granular or crystalline. zinc. Zinc Acetate Solubility.