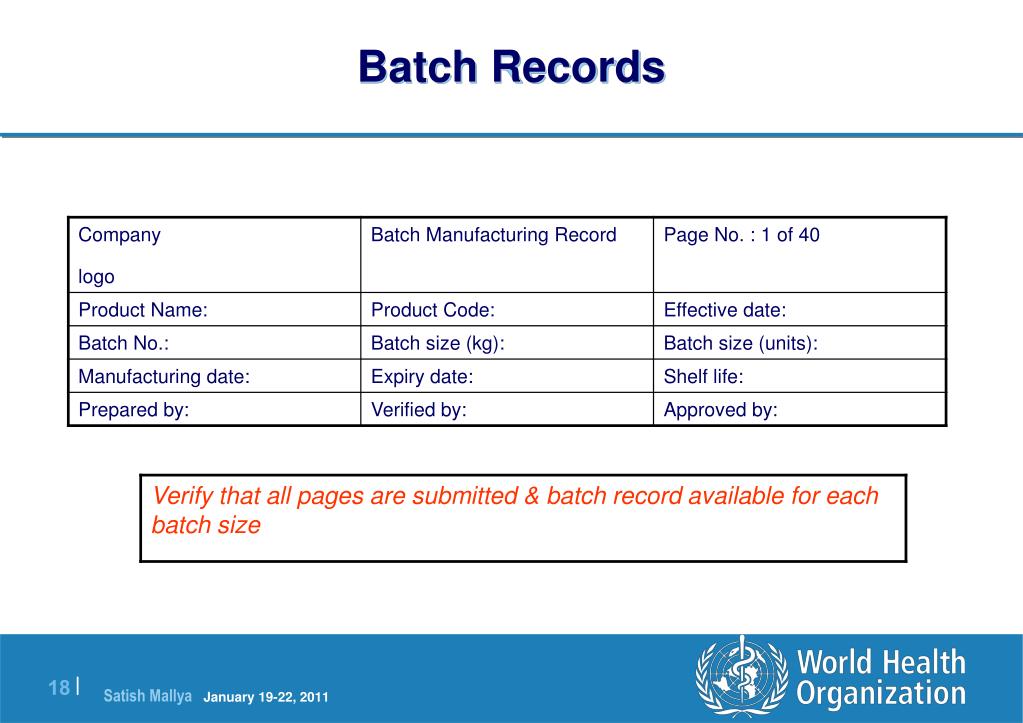
Master Batch Record Fda . (a) the master record file provides the complete procedure for manufacturing a specific product, setting. for drug products, a master batch record covering the entire process including original inoculum, propagation,. executed batch records for the primary batches should be included in the cmc technical section, along with a blank. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. if the batch production record is produced from a separate part of the master document, that document should. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including each batch. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. batch production and control records shall be prepared for each batch of drug product produced and shall include.
from www.slideserve.com
batch production and control records shall be prepared for each batch of drug product produced and shall include complete. executed batch records for the primary batches should be included in the cmc technical section, along with a blank. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. for drug products, a master batch record covering the entire process including original inoculum, propagation,. batch production and control records shall be prepared for each batch of drug product produced and shall include. if the batch production record is produced from a separate part of the master document, that document should. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including each batch. (a) the master record file provides the complete procedure for manufacturing a specific product, setting.
PPT 32. Assessing Production Documents executed and master records
Master Batch Record Fda (a) the master record file provides the complete procedure for manufacturing a specific product, setting. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including each batch. for drug products, a master batch record covering the entire process including original inoculum, propagation,. if the batch production record is produced from a separate part of the master document, that document should. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. batch production and control records shall be prepared for each batch of drug product produced and shall include. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. executed batch records for the primary batches should be included in the cmc technical section, along with a blank. (a) the master record file provides the complete procedure for manufacturing a specific product, setting.
From www.youtube.com
Batch Formula Record and Master Formula Record YouTube Master Batch Record Fda (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. if the batch production record is produced from a separate part of the master document, that document should. for drug products, a master batch record covering the entire process including original inoculum, propagation,. batch production and control records. Master Batch Record Fda.
From tutore.org
Master Batch Record Template Master Batch Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include complete. batch production and control records shall be prepared for each batch of drug product produced and shall include. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) to assure uniformity. Master Batch Record Fda.
From academy.qualitysystems.it
Il Master Batch Record Academy QS Master Batch Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include. for drug products, a master batch record covering the entire process including original inoculum, propagation,. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. (a) to assure uniformity. Master Batch Record Fda.
From www.youtube.com
Quality Assurance Batch Formula Record and Master Formula Record Master Batch Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include complete. batch production and control records shall be prepared for each batch of drug product produced and shall include. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including each batch.. Master Batch Record Fda.
From academy.qualitysystems.it
Il Master Batch Record Academy QS Master Batch Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include. executed batch records for the primary batches should be included in the cmc technical section, along with a blank. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. if. Master Batch Record Fda.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Master Batch Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. (a). Master Batch Record Fda.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Master Batch Record Fda (a) the master record file provides the complete procedure for manufacturing a specific product, setting. for drug products, a master batch record covering the entire process including original inoculum, propagation,. batch production and control records shall be prepared for each batch of drug product produced and shall include. if the batch production record is produced from. Master Batch Record Fda.
From amplelogic.com
What is Master Batch Record (MBR)? Master Batch Record Fda executed batch records for the primary batches should be included in the cmc technical section, along with a blank. if the batch production record is produced from a separate part of the master document, that document should. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. . Master Batch Record Fda.
From www.slideshare.net
Mfr Master Batch Record Fda (a) the master record file provides the complete procedure for manufacturing a specific product, setting. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including each batch. if the batch production record is produced from a separate part of the master document, that document should. batch production and. Master Batch Record Fda.
From www.mastercontrol.com
Master Production Records MasterControl Master Batch Record Fda executed batch records for the primary batches should be included in the cmc technical section, along with a blank. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) to assure. Master Batch Record Fda.
From dxotibwbh.blob.core.windows.net
Master Batch Record Eudralex at Freda Fontaine blog Master Batch Record Fda (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. (a) the master record file provides the complete procedure for manufacturing a specific product, setting. executed batch records for the primary batches should be included in the cmc technical section, along with a blank. batch production and control. Master Batch Record Fda.
From ciqa.net
What is a Master Batch Record (MBR) Versus a Batch Record (BR). Master Batch Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include. for drug products, a master batch record covering the entire process including original inoculum, propagation,. if the batch production record is produced from a separate part of the master document, that document should. executed batch records for the. Master Batch Record Fda.
From scicord.com
Batch Records increase efficiency and compliance SciCord Master Batch Record Fda (a) to assure uniformity from batch to batch, master production and control records for each drug product, including each batch. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. executed batch records for the primary batches should be included in the cmc technical section, along with a blank.. Master Batch Record Fda.
From weeverapps.com
Master Batch Records Weever Master Batch Record Fda executed batch records for the primary batches should be included in the cmc technical section, along with a blank. batch production and control records shall be prepared for each batch of drug product produced and shall include. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. . Master Batch Record Fda.
From www.plm.automation.siemens.com
What is a master batch record Siemens Software Master Batch Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include. if the batch production record is produced from a separate part of the master document, that document should. (a) the master record file provides the complete procedure for manufacturing a specific product, setting. batch production and control records. Master Batch Record Fda.
From farmasiindustri.com
Batch Record di Industri Farmasi FARMASI INDUSTRI Master Batch Record Fda for drug products, a master batch record covering the entire process including original inoculum, propagation,. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. batch production and control records shall be prepared for each batch of drug product produced and shall include. (a) to assure uniformity. Master Batch Record Fda.
From www.slideserve.com
PPT Role of FDA PowerPoint Presentation, free download ID5627662 Master Batch Record Fda (a) to assure uniformity from batch to batch, master production and control records for each drug product, including each batch. for drug products, a master batch record covering the entire process including original inoculum, propagation,. if the batch production record is produced from a separate part of the master document, that document should. batch production and. Master Batch Record Fda.
From www.slideserve.com
PPT MANUFACTURING DOCUMENTS PowerPoint Presentation, free download Master Batch Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include complete. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. batch production and control records shall be prepared for each batch of drug product produced and shall include. if. Master Batch Record Fda.
From manufacturing-software-blog.mrpeasy.com
How to Keep Perfect Batch Records? MRPeasy Master Batch Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include complete. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. (a) the master record. Master Batch Record Fda.
From old.sermitsiaq.ag
Master Batch Record Template Master Batch Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include. executed batch records for the primary batches should be included in the cmc technical section, along with a blank. for drug products, a master batch record covering the entire process including original inoculum, propagation,. batch production and control. Master Batch Record Fda.
From sgsystemsglobal.com
Electronic Batch Record (EBR) Batch Manufacturing Record (MBR) Master Batch Record Fda executed batch records for the primary batches should be included in the cmc technical section, along with a blank. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. (a) the master record file provides the complete procedure for manufacturing a specific product, setting. if the batch production. Master Batch Record Fda.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Master Batch Record Fda (a) the master record file provides the complete procedure for manufacturing a specific product, setting. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. batch production and control records shall be prepared for each batch of drug product produced and shall include. executed batch records for. Master Batch Record Fda.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Master Batch Record Fda for drug products, a master batch record covering the entire process including original inoculum, propagation,. executed batch records for the primary batches should be included in the cmc technical section, along with a blank. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. (a) to assure. Master Batch Record Fda.
From www.datacor.com
How to Prepare a Batch Manufacturing Record & Free Template (2022) Master Batch Record Fda (a) to assure uniformity from batch to batch, master production and control records for each drug product, including each batch. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. for drug products, a master batch record covering the entire process including original inoculum, propagation,. batch production and. Master Batch Record Fda.
From datamyte.com
Master Batch Record Your Essential Guide DataMyte Master Batch Record Fda (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. for drug products, a master batch record covering the entire process including original inoculum, propagation,. if the batch production. Master Batch Record Fda.
From www.slideserve.com
PPT MANUFACTURING DOCUMENTS PowerPoint Presentation, free download Master Batch Record Fda if the batch production record is produced from a separate part of the master document, that document should. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. (a). Master Batch Record Fda.
From www.slideserve.com
PPT 32. Assessing Production Documents executed and master records Master Batch Record Fda (a) the master record file provides the complete procedure for manufacturing a specific product, setting. for drug products, a master batch record covering the entire process including original inoculum, propagation,. if the batch production record is produced from a separate part of the master document, that document should. executed batch records for the primary batches should. Master Batch Record Fda.
From dxotibwbh.blob.core.windows.net
Master Batch Record Eudralex at Freda Fontaine blog Master Batch Record Fda (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including each batch. batch production and control records shall be prepared for each batch of drug product produced and shall include. . Master Batch Record Fda.
From es.scribd.com
Check List of Batch Record Production And Manufacturing Industrial Master Batch Record Fda if the batch production record is produced from a separate part of the master document, that document should. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) the master record file provides the complete procedure for manufacturing a specific product, setting. (a) to assure uniformity from batch to batch,. Master Batch Record Fda.
From www.slideshare.net
GMP Dietary Supplement Manufacturing Master Batch Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. (a) the master record file provides the complete procedure for manufacturing a specific product, setting. (a) to assure uniformity. Master Batch Record Fda.
From www.youtube.com
BMR Batch manufacturing record BMR Forms Pharma YouTube Master Batch Record Fda executed batch records for the primary batches should be included in the cmc technical section, along with a blank. batch production and control records shall be prepared for each batch of drug product produced and shall include. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. if. Master Batch Record Fda.
From manoxblog.com
Master Electronic Batch Records InstantGMP™ M A N O X B L O G Master Batch Record Fda executed batch records for the primary batches should be included in the cmc technical section, along with a blank. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including each batch. batch production. Master Batch Record Fda.
From scigeniq.com
Understanding Electronic Batch Records Scigeniq Master Batch Record Fda batch production and control records shall be prepared for each batch of drug product produced and shall include. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including each batch. if the batch production record is produced from a separate part of the master document, that document should. . Master Batch Record Fda.
From mavink.com
Batch Production Record Templates Master Batch Record Fda (a) to assure uniformity from batch to batch, master production and control records for each drug product, including. batch production and control records shall be prepared for each batch of drug product produced and shall include complete. for drug products, a master batch record covering the entire process including original inoculum, propagation,. if the batch production. Master Batch Record Fda.
From tutore.org
Master Batch Record Template Master Batch Record Fda if the batch production record is produced from a separate part of the master document, that document should. for drug products, a master batch record covering the entire process including original inoculum, propagation,. (a) to assure uniformity from batch to batch, master production and control records for each drug product, including each batch. batch production and. Master Batch Record Fda.