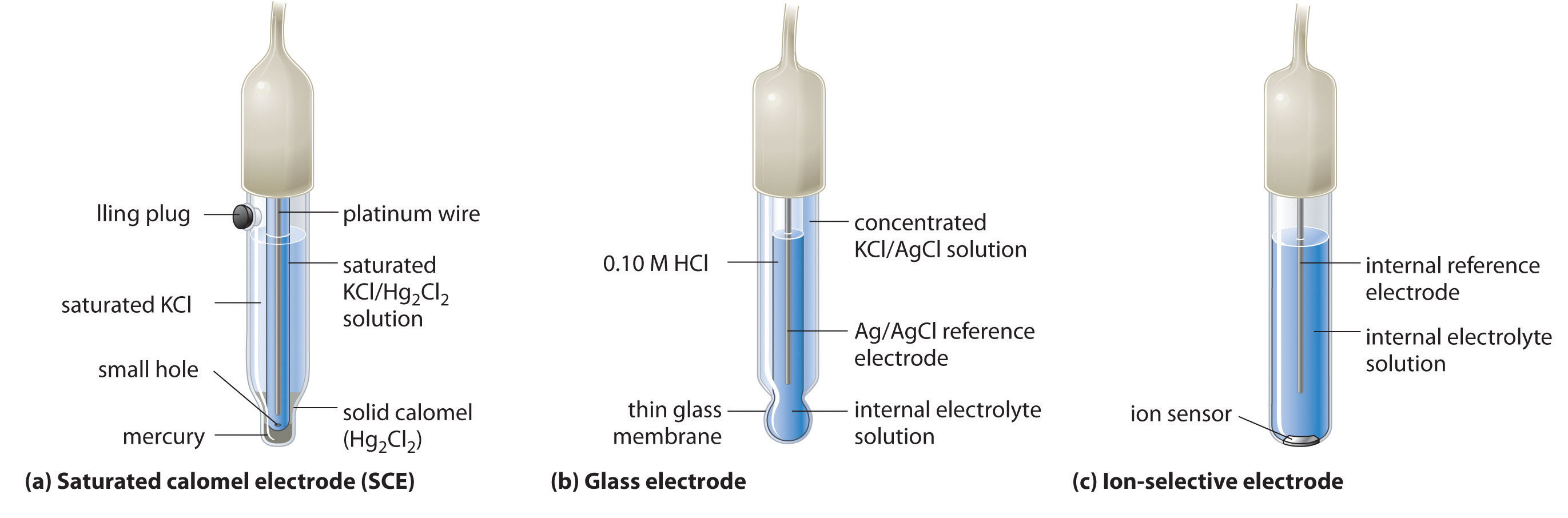
What Is The Purpose Of A Reference Electrode . the electrode potential of any other electrode that can be measured is called a reference electrode. the purpose of the auxiliary electrode (ae) is to provide a pathway for current to flow in the electrochemical cell without passing significant. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. reference electrodes are necessary to control the potential of a working electrode (e.g., during.
from chem.libretexts.org
reference electrodes are necessary to control the potential of a working electrode (e.g., during. the purpose of the auxiliary electrode (ae) is to provide a pathway for current to flow in the electrochemical cell without passing significant. the electrode potential of any other electrode that can be measured is called a reference electrode. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode.
Standard Potentials Chemistry LibreTexts
What Is The Purpose Of A Reference Electrode in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. the electrode potential of any other electrode that can be measured is called a reference electrode. the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; reference electrodes are necessary to control the potential of a working electrode (e.g., during. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the purpose of the auxiliary electrode (ae) is to provide a pathway for current to flow in the electrochemical cell without passing significant.
From armingroup.com
Reference electrodes What Is The Purpose Of A Reference Electrode reference electrodes are necessary to control the potential of a working electrode (e.g., during. the purpose of the auxiliary electrode (ae) is to provide a pathway for current to flow in the electrochemical cell without passing significant. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to. What Is The Purpose Of A Reference Electrode.
From www.slideserve.com
PPT Electrochemical cells PowerPoint Presentation, free download ID What Is The Purpose Of A Reference Electrode the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to. What Is The Purpose Of A Reference Electrode.
From gaskatel.de
Reference electrode Gaskatel What Is The Purpose Of A Reference Electrode the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground. What Is The Purpose Of A Reference Electrode.
From www.researchgate.net
(A) Placement of reference electrode. (B) 1D representation of What Is The Purpose Of A Reference Electrode the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. reference electrodes are necessary to control the potential of a working electrode (e.g., during. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the. What Is The Purpose Of A Reference Electrode.
From www.mdpi.com
Encyclopedia Free FullText Reference Electrodes What Is The Purpose Of A Reference Electrode in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the purpose of the auxiliary electrode (ae) is to provide a pathway for current to flow in the electrochemical cell without passing significant. reference electrodes are necessary to control the potential of a working electrode (e.g., during. the ideal reference electrode. What Is The Purpose Of A Reference Electrode.
From www.researchgate.net
Design of the reference electrodes with the use of a solid electrolyte What Is The Purpose Of A Reference Electrode the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry. What Is The Purpose Of A Reference Electrode.
From byjus.com
79.What is reference electrode What Is The Purpose Of A Reference Electrode the electrode potential of any other electrode that can be measured is called a reference electrode. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. the purpose of the auxiliary electrode (ae) is to provide a pathway for current to flow. What Is The Purpose Of A Reference Electrode.
From www.youtube.com
Lesson 57 Reference Electrodes YouTube What Is The Purpose Of A Reference Electrode in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; the purpose of the auxiliary electrode (ae). What Is The Purpose Of A Reference Electrode.
From studylib.net
Reference Electrodes What Is The Purpose Of A Reference Electrode the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect. What Is The Purpose Of A Reference Electrode.
From www.researchgate.net
"Open" and "close" designs of the reference electrode compartment of What Is The Purpose Of A Reference Electrode the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. reference electrodes are necessary. What Is The Purpose Of A Reference Electrode.
From www.vedantu.com
Define the reference electrode. What Is The Purpose Of A Reference Electrode the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. the purpose of the auxiliary electrode (ae) is to provide a pathway for current to flow in the electrochemical cell without passing significant. in potentiometry, those two electrodes are generally called the indicator electrode and. What Is The Purpose Of A Reference Electrode.
From askfilo.com
29. Define reference electrode. Write the functions of salt bridge. Draw What Is The Purpose Of A Reference Electrode in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the electrode potential of any other electrode that can be measured is called a reference electrode. reference electrodes are necessary to control the potential of a working electrode (e.g., during. the purpose of the auxiliary electrode (ae) is to provide a. What Is The Purpose Of A Reference Electrode.
From www.gamry.com
Reference Electrodes Saturated CalomelSilver ChlorideMercury Oxide What Is The Purpose Of A Reference Electrode the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. reference electrodes are necessary to control the potential of a. What Is The Purpose Of A Reference Electrode.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts What Is The Purpose Of A Reference Electrode in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; the ideal reference electrode provides a stable,. What Is The Purpose Of A Reference Electrode.
From www.slideserve.com
PPT Uses of Reference Electrodes for various purposes PowerPoint What Is The Purpose Of A Reference Electrode the electrode potential of any other electrode that can be measured is called a reference electrode. reference electrodes are necessary to control the potential of a working electrode (e.g., during. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the ag/agcl reference electrode is a commonly used reference electrode in. What Is The Purpose Of A Reference Electrode.
From www.allaboutcircuits.com
pH Measurement Electrical Instrumentation Signals Electronics Textbook What Is The Purpose Of A Reference Electrode the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the purpose of the auxiliary electrode (ae) is to provide a pathway for current to flow in the electrochemical cell without passing significant. reference electrodes are necessary to control. What Is The Purpose Of A Reference Electrode.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry What Is The Purpose Of A Reference Electrode reference electrodes are necessary to control the potential of a working electrode (e.g., during. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the purpose. What Is The Purpose Of A Reference Electrode.
From www.scribd.com
Reference Electrode PDF What Is The Purpose Of A Reference Electrode the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. reference electrodes are necessary to control the potential of a working electrode (e.g., during. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the electrode potential of any. What Is The Purpose Of A Reference Electrode.
From www.mdpi.com
Encyclopedia Free FullText Reference Electrodes What Is The Purpose Of A Reference Electrode the electrode potential of any other electrode that can be measured is called a reference electrode. the purpose of the auxiliary electrode (ae) is to provide a pathway for current to flow in the electrochemical cell without passing significant. the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; in potentiometry, those two electrodes. What Is The Purpose Of A Reference Electrode.
From www.researchgate.net
(PDF) Reference Electrodes What Is The Purpose Of A Reference Electrode the purpose of the auxiliary electrode (ae) is to provide a pathway for current to flow in the electrochemical cell without passing significant. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. the purpose of a reference electrode is to complete. What Is The Purpose Of A Reference Electrode.
From www.researchgate.net
Schematic description for reference electrode connection to MFC What Is The Purpose Of A Reference Electrode the electrode potential of any other electrode that can be measured is called a reference electrode. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. the ag/agcl reference. What Is The Purpose Of A Reference Electrode.
From www.slideserve.com
PPT 141 Reference Electrodes PowerPoint Presentation, free download What Is The Purpose Of A Reference Electrode the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. reference electrodes are necessary to control the potential of a working electrode (e.g., during. the electrode potential of any other electrode that can be measured is called a reference electrode. the. What Is The Purpose Of A Reference Electrode.
From chem.libretexts.org
23.1 Reference Electrodes Chemistry LibreTexts What Is The Purpose Of A Reference Electrode reference electrodes are necessary to control the potential of a working electrode (e.g., during. the electrode potential of any other electrode that can be measured is called a reference electrode. the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; the purpose of a reference electrode is to complete the electrical circuit and provide. What Is The Purpose Of A Reference Electrode.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision What Is The Purpose Of A Reference Electrode the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; reference electrodes are necessary to control the potential of a working electrode (e.g., during. the purpose of the auxiliary electrode (ae) is to provide a pathway for current to flow in the electrochemical cell without passing significant. in potentiometry, those two electrodes are generally. What Is The Purpose Of A Reference Electrode.
From www.hamiltoncompany.com
The pH Reference Electrode Process Analytics Hamilton Company What Is The Purpose Of A Reference Electrode reference electrodes are necessary to control the potential of a working electrode (e.g., during. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the. What Is The Purpose Of A Reference Electrode.
From semcouniversity.com
How the three electrode system works Semco University Semco What Is The Purpose Of A Reference Electrode in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. the electrode potential of any other electrode. What Is The Purpose Of A Reference Electrode.
From studylib.net
Reference Electrodes What Is The Purpose Of A Reference Electrode reference electrodes are necessary to control the potential of a working electrode (e.g., during. the electrode potential of any other electrode that can be measured is called a reference electrode. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the ag/agcl reference electrode is a commonly used reference electrode in. What Is The Purpose Of A Reference Electrode.
From dxotaboqx.blob.core.windows.net
Medical Electrodes Types at Marian Mask blog What Is The Purpose Of A Reference Electrode in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the purpose of the auxiliary electrode (ae) is to provide a pathway for current to flow in the electrochemical cell without passing significant. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell. What Is The Purpose Of A Reference Electrode.
From www.researchgate.net
"Open" and "close" designs of the reference electrode compartment of What Is The Purpose Of A Reference Electrode the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. in potentiometry, those two electrodes are generally called the indicator. What Is The Purpose Of A Reference Electrode.
From www.researchgate.net
Tripolar cell (WE working electrode, Re1 reference electrode and CE What Is The Purpose Of A Reference Electrode the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. the electrode potential of any other electrode that can be. What Is The Purpose Of A Reference Electrode.
From mungfali.com
Reference Electrode Diagram What Is The Purpose Of A Reference Electrode the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. the electrode potential of any other electrode that can be measured is called a reference electrode. reference electrodes are necessary to control the potential of a working electrode (e.g., during. the. What Is The Purpose Of A Reference Electrode.
From www.numerade.com
SOLVED 4.A) What is a reference electrode? Explain the purpose of What Is The Purpose Of A Reference Electrode the electrode potential of any other electrode that can be measured is called a reference electrode. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. . What Is The Purpose Of A Reference Electrode.
From www.researchgate.net
Working electrode. Reference Electrode The reference electrode used What Is The Purpose Of A Reference Electrode in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. reference electrodes are necessary to control the potential of a working electrode (e.g., during. the electrode potential of any other electrode that can be measured is called a reference electrode. the ideal reference electrode provides a stable, known potential so that. What Is The Purpose Of A Reference Electrode.
From www.lambdasystem.pl
Reference electrodes Electrodes ELECTROCHEMISTRY ACCESSORIES What Is The Purpose Of A Reference Electrode the electrode potential of any other electrode that can be measured is called a reference electrode. the ag/agcl reference electrode is a commonly used reference electrode in electrochemistry; in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the ideal reference electrode provides a stable, known potential so that we can. What Is The Purpose Of A Reference Electrode.
From www.youtube.com
Reference Electrodes YouTube What Is The Purpose Of A Reference Electrode the electrode potential of any other electrode that can be measured is called a reference electrode. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to carry out electrochemical. the purpose of the auxiliary electrode (ae) is to provide a pathway for current to flow in the electrochemical. What Is The Purpose Of A Reference Electrode.