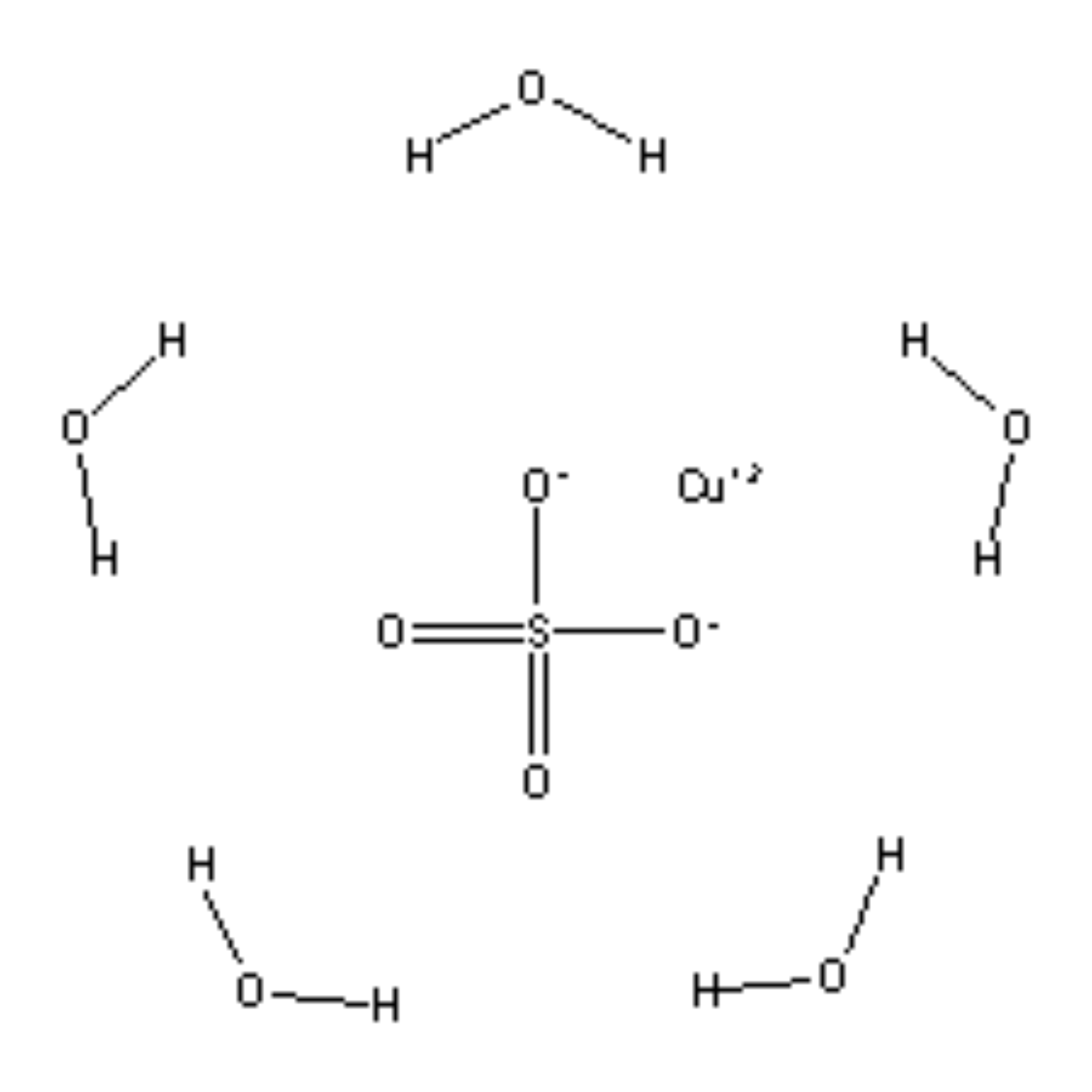
Copper Ii Sulfate Electron Configuration . $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. Electronic configuration of $\ce{co}$ is as follows: How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Justify the observed charge of ions to their electronic configuration. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. Determine the electron configuration of ions.
from www.rcilabscan.com
If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. Determine the electron configuration of ions. Justify the observed charge of ions to their electronic configuration. Electronic configuration of $\ce{co}$ is as follows: $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$.
Copper (II) Sulfate Pentahydrate, AR RCI LABSCAN LIMITED (EN)
Copper Ii Sulfate Electron Configuration When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. Electronic configuration of $\ce{co}$ is as follows: When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. Determine the electron configuration of ions. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Justify the observed charge of ions to their electronic configuration. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Ii Sulfate Electron Configuration How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Justify the observed charge of ions to their electronic configuration. When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. Electronic configuration of $\ce{co}$ is as follows: If. Copper Ii Sulfate Electron Configuration.
From www.youtube.com
Is CuSO4, Copper (II) sulfate, Ionic or Covalent? YouTube Copper Ii Sulfate Electron Configuration $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. Determine the electron configuration of ions. How to write the electron. Copper Ii Sulfate Electron Configuration.
From www.t3db.ca
T3DB Copper(II) sulfate Copper Ii Sulfate Electron Configuration Determine the electron configuration of ions. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Electronic configuration of $\ce{co}$ is as. Copper Ii Sulfate Electron Configuration.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Ii Sulfate Electron Configuration How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Determine the electron configuration of ions. When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. Electronic configuration of $\ce{co}$ is as follows: $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. Justify the observed charge of. Copper Ii Sulfate Electron Configuration.
From www.youtube.com
Electrolysis Of Copper(ii) Sulphate Using Copper Electrodes YouTube Copper Ii Sulfate Electron Configuration How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Determine the electron configuration of ions. Justify the observed charge of ions to their electronic configuration. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are. Copper Ii Sulfate Electron Configuration.
From sciencenotes.org
List of Electron Configurations of Elements Copper Ii Sulfate Electron Configuration Electronic configuration of $\ce{co}$ is as follows: Justify the observed charge of ions to their electronic configuration. Determine the electron configuration of ions. When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the. Copper Ii Sulfate Electron Configuration.
From www.sciencephoto.com
Copper (II) sulphate forms Stock Image C004/7693 Science Photo Copper Ii Sulfate Electron Configuration Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Electronic configuration of $\ce{co}$ is as follows: If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. Determine the electron configuration. Copper Ii Sulfate Electron Configuration.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper Ii Sulfate Electron Configuration $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. Electronic configuration of $\ce{co}$ is as follows: Justify the observed charge of ions to their electronic configuration. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. Determine the electron configuration of. Copper Ii Sulfate Electron Configuration.
From www.youtube.com
Lewis Structure of CuS, copper (II) sulfide YouTube Copper Ii Sulfate Electron Configuration Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Justify the observed charge of ions to their electronic configuration. Electronic configuration of $\ce{co}$ is as follows: If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. Determine. Copper Ii Sulfate Electron Configuration.
From www.alamy.com
Copper(II) sulfate with molecular structure. Chemical ingredient used Copper Ii Sulfate Electron Configuration How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Electronic configuration of $\ce{co}$ is as follows: Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Determine the electron configuration of ions. $1s^2\. Copper Ii Sulfate Electron Configuration.
From chem.libretexts.org
Chapter 2.7 Electronic Structure of the Transition Metals Chemistry Copper Ii Sulfate Electron Configuration Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Justify the observed charge of ions to their electronic configuration. When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. Electronic configuration of $\ce{co}$ is as follows: How to write the electron configuration for copper (cu, cu+, and cu2+) in order. Copper Ii Sulfate Electron Configuration.
From www.wikidoc.org
Copper(II) sulfate wikidoc Copper Ii Sulfate Electron Configuration Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. Electronic configuration of $\ce{co}$ is as follows: When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. Justify the observed charge of ions to their electronic configuration. If white light (ordinary sunlight, for example). Copper Ii Sulfate Electron Configuration.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Copper Ii Sulfate Electron Configuration $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. Justify the observed charge of ions to their electronic configuration. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Electronic. Copper Ii Sulfate Electron Configuration.
From www.alamy.com
Copper(II) sulfate with molecular structure. Chemical ingredient used Copper Ii Sulfate Electron Configuration Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Justify the observed charge of ions to their electronic configuration. When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. Determine the electron configuration of ions. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in. Copper Ii Sulfate Electron Configuration.
From proper-cooking.info
Copper Electron Dot Diagram Copper Ii Sulfate Electron Configuration Justify the observed charge of ions to their electronic configuration. $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. Electronic configuration of $\ce{co}$ is as follows: Determine the electron configuration of ions. When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the. Copper Ii Sulfate Electron Configuration.
From www.glentham.com
Copper(II) sulfate, anhydrous, 98 (CAS 7758987) Glentham Life Sciences Copper Ii Sulfate Electron Configuration If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. Justify the observed charge of ions to their electronic configuration. Determine the electron configuration of ions. $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. Copper (ii) sulfate, also known as. Copper Ii Sulfate Electron Configuration.
From diagramweb.net
Strontium Orbital Diagram Copper Ii Sulfate Electron Configuration How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths. Copper Ii Sulfate Electron Configuration.
From molekula.com
Purchase Copper(II) sulfate pentahydrate [7758998] online • Catalog Copper Ii Sulfate Electron Configuration Determine the electron configuration of ions. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Electronic configuration of $\ce{co}$ is as follows: Justify the observed charge of ions to their electronic configuration. $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. If white light (ordinary sunlight, for example) passes through copper(ii). Copper Ii Sulfate Electron Configuration.
From www.vectorstock.com
Symbol and electron diagram for copper Royalty Free Vector Copper Ii Sulfate Electron Configuration Determine the electron configuration of ions. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the. Copper Ii Sulfate Electron Configuration.
From www.sciencephoto.com
Copper (II) Sulphate Stock Image C036/3376 Science Photo Library Copper Ii Sulfate Electron Configuration Electronic configuration of $\ce{co}$ is as follows: How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Determine the electron configuration of ions. $1s^2\. Copper Ii Sulfate Electron Configuration.
From byjus.com
Explain the electrolysis of copper sulphate solution with the help of a Copper Ii Sulfate Electron Configuration Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Determine the electron configuration of ions. Electronic configuration of $\ce{co}$ is as follows: How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. If. Copper Ii Sulfate Electron Configuration.
From www.youtube.com
How to find Protons & Electrons for Cu+ and Cu2+ (Copper II and III Copper Ii Sulfate Electron Configuration $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. Electronic configuration of $\ce{co}$ is. Copper Ii Sulfate Electron Configuration.
From izayahmeowcrosby.blogspot.com
Electronic Configuration of Copper Copper Ii Sulfate Electron Configuration If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Justify the observed charge of ions to their electronic configuration. Electronic configuration. Copper Ii Sulfate Electron Configuration.
From www.alamy.com
Copper(II) sulfate with molecular structure. Chemical ingredient used Copper Ii Sulfate Electron Configuration $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. Electronic configuration of $\ce{co}$ is. Copper Ii Sulfate Electron Configuration.
From www.pngwing.com
Copper(II) sulfate Crystal structure Chemical compound, salt, natural Copper Ii Sulfate Electron Configuration Justify the observed charge of ions to their electronic configuration. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Electronic configuration of $\ce{co}$ is as follows: How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need. Copper Ii Sulfate Electron Configuration.
From ar.inspiredpencil.com
Copper Ii Sulfate Pentahydrate Anhydrous Copper Ii Sulfate Electron Configuration Justify the observed charge of ions to their electronic configuration. $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Electronic configuration of $\ce{co}$ is as follows: How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write. Copper Ii Sulfate Electron Configuration.
From www.youtube.com
How to Write the Formula for Copper (II) sulfate YouTube Copper Ii Sulfate Electron Configuration Justify the observed charge of ions to their electronic configuration. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. Determine the electron configuration of ions. When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. Electronic configuration of $\ce{co}$ is as. Copper Ii Sulfate Electron Configuration.
From www.alamy.com
3D image of Copper II sulfate skeletal formula molecular chemical Copper Ii Sulfate Electron Configuration $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Justify the observed charge of ions to their electronic configuration. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. How. Copper Ii Sulfate Electron Configuration.
From www.rcilabscan.com
Copper (II) Sulfate Pentahydrate, AR RCI LABSCAN LIMITED (EN) Copper Ii Sulfate Electron Configuration Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Electronic configuration of $\ce{co}$ is as follows: Determine the electron configuration of ions. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Justify. Copper Ii Sulfate Electron Configuration.
From www.reddit.com
Copper(II) sulfate r/MineralPorn Copper Ii Sulfate Electron Configuration $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. Determine the electron configuration of ions. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are. Copper Ii Sulfate Electron Configuration.
From elchoroukhost.net
Copper Periodic Table Protons Neutrons And Electrons Elcho Table Copper Ii Sulfate Electron Configuration If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. Determine the electron configuration of ions. Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. Justify the observed charge of. Copper Ii Sulfate Electron Configuration.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper Ii Sulfate Electron Configuration Justify the observed charge of ions to their electronic configuration. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. Determine the electron configuration of ions. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Copper Ii Sulfate Electron Configuration.
From ar.inspiredpencil.com
Electron Configuration Of Copper Copper Ii Sulfate Electron Configuration Electronic configuration of $\ce{co}$ is as follows: Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are absorbed by the solution. Justify the observed charge of ions to their electronic configuration. $1s^2\. Copper Ii Sulfate Electron Configuration.
From organicful44.blogspot.com
copper orbital diagram Organicful Copper Ii Sulfate Electron Configuration Determine the electron configuration of ions. When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. Electronic configuration of $\ce{co}$ is as follows: Copper (ii) sulfate, also known as cupric sulfate, is a widely used chemical compound with the chemical formula cuso 4. If white light (ordinary sunlight, for example) passes through copper(ii) sulfate solution, some wavelengths in the light are. Copper Ii Sulfate Electron Configuration.
From www.youtube.com
Color of Copper (II) sulfate [hydrous and anhydrous] YouTube Copper Ii Sulfate Electron Configuration When cobalt loses 2 electrons to become $\ce{co^{2+}}$ it. $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 4s^2\ 3d^7$. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Justify the observed charge of ions to their electronic configuration. Electronic configuration of $\ce{co}$ is as follows: Determine. Copper Ii Sulfate Electron Configuration.