Chlorine Displace Bromine Equation . Therefore, chlorine will displace the bromine in. Bromine only displaces iodine from. learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. chlorine displaces bromine from potassium bromide and iodine from potassium iodide. because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity is therefore: chlorine is above bromine in the group 7 column, which means that chlorine is more reactive than bromine. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. video and resources investigating the displacement reactions between.
from www.numerade.com
Bromine only displaces iodine from. learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. chlorine is above bromine in the group 7 column, which means that chlorine is more reactive than bromine. Therefore, chlorine will displace the bromine in. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity is therefore: chlorine displaces bromine from potassium bromide and iodine from potassium iodide. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. video and resources investigating the displacement reactions between.
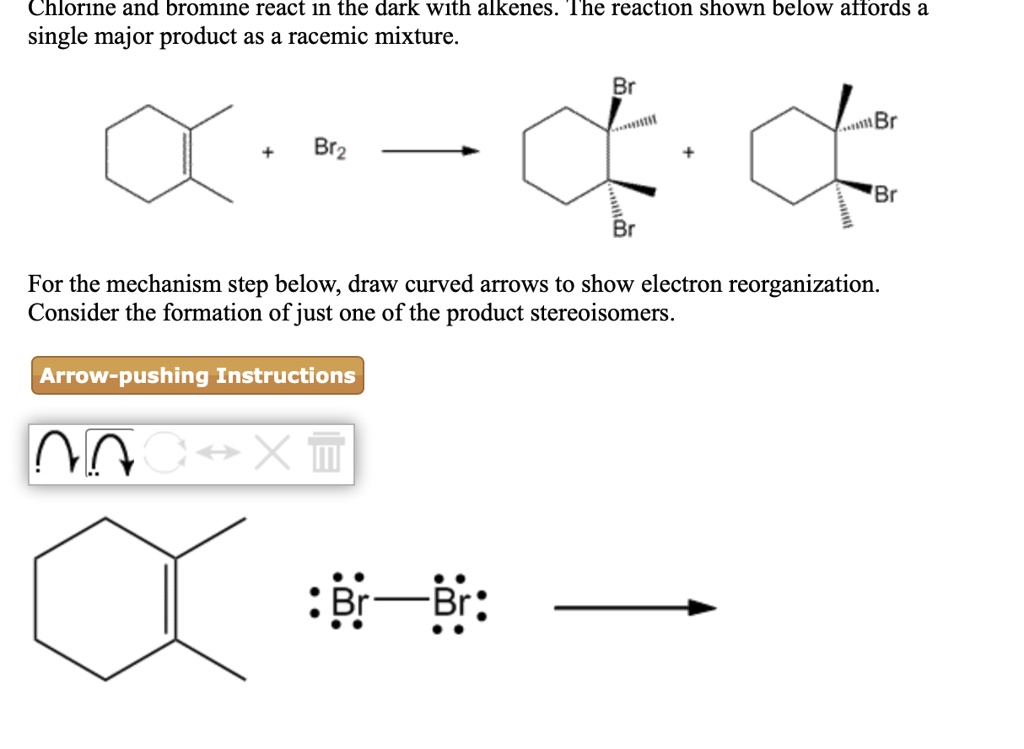
SOLVED Chlorine and bromine react In the dark WIth alkenes The
Chlorine Displace Bromine Equation video and resources investigating the displacement reactions between. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Bromine only displaces iodine from. learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. video and resources investigating the displacement reactions between. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity is therefore: chlorine is above bromine in the group 7 column, which means that chlorine is more reactive than bromine. Therefore, chlorine will displace the bromine in. chlorine displaces bromine from potassium bromide and iodine from potassium iodide.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Chlorine Displace Bromine Equation learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. Therefore, chlorine will displace the bromine in. chlorine displaces bromine from potassium bromide and iodine from potassium iodide. because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Bromine only displaces iodine from.. Chlorine Displace Bromine Equation.
From www.doubtnut.com
Bromine can displace chlorine from its salt solution. Chlorine Displace Bromine Equation learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. chlorine displaces bromine from potassium bromide and iodine from potassium iodide. Therefore, chlorine will displace the bromine in. learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. chlorine is above. Chlorine Displace Bromine Equation.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Chlorine Displace Bromine Equation chlorine displaces bromine from potassium bromide and iodine from potassium iodide. Bromine only displaces iodine from. Therefore, chlorine will displace the bromine in. video and resources investigating the displacement reactions between. learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the. Chlorine Displace Bromine Equation.
From www.numerade.com
SOLVED Potassium bromide + chlorine = potassium chloride + bromine Chlorine Displace Bromine Equation learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. chlorine is above bromine in the group 7 column, which means that chlorine is more reactive than bromine. learn how halogens. Chlorine Displace Bromine Equation.
From www.myxxgirl.com
What Is A Balanced Equation For The Reaction Between Chlorine And My Chlorine Displace Bromine Equation Therefore, chlorine will displace the bromine in. learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. chlorine is above bromine in the group 7 column, which means that chlorine is more reactive than bromine. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. Cl 2 (aq). Chlorine Displace Bromine Equation.
From www.tes.com
Lesson Halogen Displacement GCSE Edexcel 91 Teaching Resources Chlorine Displace Bromine Equation in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. Bromine only displaces iodine from. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity. Chlorine Displace Bromine Equation.
From www.numerade.com
SOLVED (ii) Aniline reacts with bromine water in aqueous medium (only Chlorine Displace Bromine Equation learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Therefore, chlorine will displace the bromine in. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity is therefore: learn. Chlorine Displace Bromine Equation.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Chlorine Displace Bromine Equation because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. chlorine displaces bromine from potassium bromide and iodine from potassium iodide.. Chlorine Displace Bromine Equation.
From www.numerade.com
SOLVEDThe first preparation of chlorine was similar to the synthesis Chlorine Displace Bromine Equation Bromine only displaces iodine from. video and resources investigating the displacement reactions between. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity is. Chlorine Displace Bromine Equation.
From www.chegg.com
Solved Chlorine and bromine react in the dark with alkenes. Chlorine Displace Bromine Equation learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. video and resources investigating the displacement reactions between. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. Bromine only. Chlorine Displace Bromine Equation.
From www.chegg.com
Solved Chlorine and bromine react in the dark with alkenes. Chlorine Displace Bromine Equation in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. chlorine is above bromine in the group 7 column, which means that chlorine is more reactive than bromine. learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. . Chlorine Displace Bromine Equation.
From gcse-revision-chemistry.blogspot.com
Beccy's Chemistry Revision 2018 Section 2 c) Summary Chlorine Displace Bromine Equation video and resources investigating the displacement reactions between. chlorine displaces bromine from potassium bromide and iodine from potassium iodide. learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. chlorine is above bromine in the group 7 column, which means that chlorine is more reactive than bromine. in the displacement reactions. Chlorine Displace Bromine Equation.
From www.bbc.co.uk
What is a displacement reaction? BBC Bitesize Chlorine Displace Bromine Equation in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. video and resources investigating the displacement reactions between. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity is therefore: learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. . Chlorine Displace Bromine Equation.
From exoedqopw.blob.core.windows.net
Ionization Energy Of Bromine Equation at David Taber blog Chlorine Displace Bromine Equation learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. chlorine is above bromine in the group 7 column, which means that chlorine is more reactive than bromine. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. . Chlorine Displace Bromine Equation.
From www.science-revision.co.uk
Halogen displacement reactions Chlorine Displace Bromine Equation because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. chlorine displaces bromine from potassium bromide and iodine from potassium iodide. chlorine is above bromine in the group 7 column, which means that chlorine is more reactive than. Chlorine Displace Bromine Equation.
From sciencestruck.com
Bromine Vs. Chlorine Chlorine Displace Bromine Equation Bromine only displaces iodine from. learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity is therefore: video and resources investigating the displacement reactions between.. Chlorine Displace Bromine Equation.
From www.jove.com
12874.jpg Chlorine Displace Bromine Equation because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity is therefore: Bromine only displaces iodine from. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. chlorine is above bromine in. Chlorine Displace Bromine Equation.
From studylib.net
Displacement reactions Reactions of chlorine Chlorine Displace Bromine Equation in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Therefore, chlorine will displace the bromine in. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity is therefore: because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Bromine only displaces iodine. Chlorine Displace Bromine Equation.
From www.numerade.com
SOLVED Chlorine and bromine react In the dark WIth alkenes The Chlorine Displace Bromine Equation Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity is therefore: because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. chlorine displaces bromine from potassium bromide and iodine from potassium iodide. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. Therefore,. Chlorine Displace Bromine Equation.
From www.chegg.com
Solved 9. For the reaction of sodium bromide with chlorine Chlorine Displace Bromine Equation Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity is therefore: learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. chlorine displaces bromine from potassium bromide and iodine from potassium iodide. learn how halogens displace each other from aqueous. Chlorine Displace Bromine Equation.
From www.numerade.com
SOLVEDBromine can be prepared by adding chlorine to an aqueous Chlorine Displace Bromine Equation learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. Therefore, chlorine will displace the bromine in. learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by.. Chlorine Displace Bromine Equation.
From www.researchgate.net
Analytical scheme for radiochemical purification of chlorine, bromine Chlorine Displace Bromine Equation Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity is therefore: learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. because chlorine is more. Chlorine Displace Bromine Equation.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Chlorine Displace Bromine Equation video and resources investigating the displacement reactions between. chlorine is above bromine in the group 7 column, which means that chlorine is more reactive than bromine. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. Bromine only displaces iodine from. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq). Chlorine Displace Bromine Equation.
From solvedlib.com
Chlorine and bromine react in the dark with alkenes. … SolvedLib Chlorine Displace Bromine Equation learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. Bromine only displaces iodine from. video and resources investigating the displacement reactions between. learn how halogens displace each other from aqueous. Chlorine Displace Bromine Equation.
From www.numerade.com
SOLVED Sodium chloride reacts with bromine gas, mathrm{Br}_{2}, to Chlorine Displace Bromine Equation learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. video and resources investigating the. Chlorine Displace Bromine Equation.
From www.numerade.com
SOLVEDGaseous chlorine will displace bromide ion from an aqueous Chlorine Displace Bromine Equation because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. chlorine displaces bromine from potassium bromide and iodine from potassium iodide. Bromine only displaces iodine from. Therefore, chlorine will displace the bromine in. learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. Cl 2 (aq) + 2ki(aq) → i. Chlorine Displace Bromine Equation.
From slideplayer.com
Biology Dr. Altstiel Naples American High School ppt download Chlorine Displace Bromine Equation in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. chlorine displaces bromine from potassium bromide and iodine from potassium iodide. Therefore, chlorine will displace the bromine in. learn how chlorine, bromine and iodine. Chlorine Displace Bromine Equation.
From www.docbrown.info
Free radical reaction of alkanes with bromine chlorine conditions Chlorine Displace Bromine Equation Therefore, chlorine will displace the bromine in. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. video and resources investigating the displacement reactions between. chlorine displaces bromine from potassium bromide and iodine from potassium iodide. learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. . Chlorine Displace Bromine Equation.
From londonstatus.co.uk
Why Does Chlorine Displace Bromine from Sodium Bromide? London Status Chlorine Displace Bromine Equation Therefore, chlorine will displace the bromine in. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. chlorine is above bromine in the group 7 column, which means that chlorine is more reactive than bromine. Bromine only displaces iodine from. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order. Chlorine Displace Bromine Equation.
From exoedqopw.blob.core.windows.net
Ionization Energy Of Bromine Equation at David Taber blog Chlorine Displace Bromine Equation Therefore, chlorine will displace the bromine in. Cl 2 (aq) + 2ki(aq) → i 2 (aq) + 2kcl(aq) the order of reactivity is therefore: Bromine only displaces iodine from. learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. learn how halogens displace each other from aqueous. Chlorine Displace Bromine Equation.
From socratic.org
What is the balanced chemical equation for this reaction Gaseous Chlorine Displace Bromine Equation Therefore, chlorine will displace the bromine in. Bromine only displaces iodine from. video and resources investigating the displacement reactions between. chlorine is above bromine in the group 7 column, which means that chlorine is more reactive than bromine. learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic. Chlorine Displace Bromine Equation.
From www.bbc.co.uk
What is a displacement reaction? BBC Bitesize Chlorine Displace Bromine Equation learn how to determine the order of reactivity of chlorine, bromine and iodine in group 7 of the periodic table by. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. chlorine is above bromine in the group 7 column, which means that chlorine is more reactive than bromine. . Chlorine Displace Bromine Equation.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download Chlorine Displace Bromine Equation learn how chlorine, bromine and iodine react with potassium bromide and potassium iodide solutions. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. Therefore, chlorine will displace the bromine in. because chlorine is. Chlorine Displace Bromine Equation.
From www.tessshebaylo.com
Salt Water Electrolysis Equation Tessshebaylo Chlorine Displace Bromine Equation in the displacement reactions chlorine displaces both bromine and iodine from their compounds and bromine displaces iodine. because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. chlorine displaces bromine from potassium bromide and iodine from potassium iodide.. Chlorine Displace Bromine Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Chlorine Displace Bromine Equation chlorine displaces bromine from potassium bromide and iodine from potassium iodide. Therefore, chlorine will displace the bromine in. video and resources investigating the displacement reactions between. learn how halogens displace each other from aqueous solutions of metal halides in redox reactions. because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. learn. Chlorine Displace Bromine Equation.