Magnesium Chloride And Sodium Hydroxide Formula . mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions are colourless. Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. what is the formula for barium chloride? Mgcl2 crystallizes in the cadmium chloride cdcl2 motif, which features octahedral mg centers. a novel process for obtaining magnesium from sea water involves several reactions. aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. Barium is an alkaline earth and always corms a cation of charge of [+2],. The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by.
from www.chegg.com
Mgcl2 crystallizes in the cadmium chloride cdcl2 motif, which features octahedral mg centers. a novel process for obtaining magnesium from sea water involves several reactions. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions are colourless. The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by. Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. what is the formula for barium chloride? aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. Barium is an alkaline earth and always corms a cation of charge of [+2],.
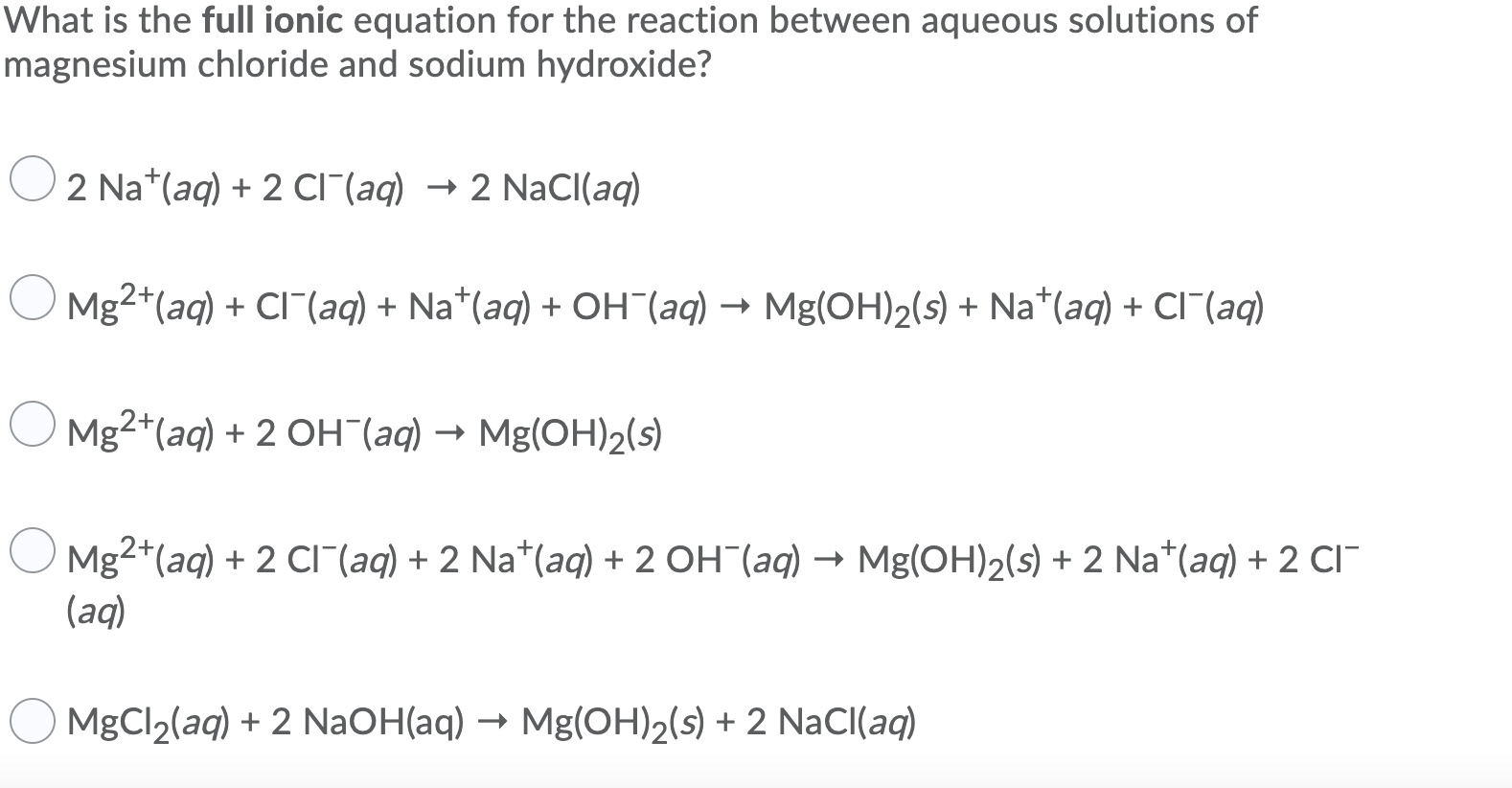
Solved What is the full ionic equation for the reaction
Magnesium Chloride And Sodium Hydroxide Formula aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. what is the formula for barium chloride? Barium is an alkaline earth and always corms a cation of charge of [+2],. Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. a novel process for obtaining magnesium from sea water involves several reactions. The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by. aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. Mgcl2 crystallizes in the cadmium chloride cdcl2 motif, which features octahedral mg centers. mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions are colourless.
From exywaygsf.blob.core.windows.net
Ammonium Phosphate And Magnesium Chloride Formula at Shroyer blog Magnesium Chloride And Sodium Hydroxide Formula Mgcl2 crystallizes in the cadmium chloride cdcl2 motif, which features octahedral mg centers. Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions are colourless. a novel process. Magnesium Chloride And Sodium Hydroxide Formula.
From www.youtube.com
How to Write the Formula for Magnesium hydroxide YouTube Magnesium Chloride And Sodium Hydroxide Formula thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. a novel process for obtaining magnesium from sea water involves several reactions. Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. what. Magnesium Chloride And Sodium Hydroxide Formula.
From ar.inspiredpencil.com
Magnesium Chloride Lewis Dot Structure Magnesium Chloride And Sodium Hydroxide Formula Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different. Magnesium Chloride And Sodium Hydroxide Formula.
From www.chegg.com
Solved (20pts) Reaction Yields Antacid Milk of Magnesia is a Magnesium Chloride And Sodium Hydroxide Formula a novel process for obtaining magnesium from sea water involves several reactions. aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. Mgcl2 +. Magnesium Chloride And Sodium Hydroxide Formula.
From www.studyxapp.com
1340 magnesium chloride and sodium hydroxide react form magnesium Magnesium Chloride And Sodium Hydroxide Formula mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions are colourless. a novel process for obtaining magnesium from sea water involves several reactions. The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by. thus, \(\ce{nacl}\) is. Magnesium Chloride And Sodium Hydroxide Formula.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Magnesium Chloride And Sodium Hydroxide Formula Barium is an alkaline earth and always corms a cation of charge of [+2],. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. . Magnesium Chloride And Sodium Hydroxide Formula.
From www.chegg.com
Solved When aqueous solutions of magnesium chloride and Magnesium Chloride And Sodium Hydroxide Formula a novel process for obtaining magnesium from sea water involves several reactions. what is the formula for barium chloride? mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions are colourless. aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide. Magnesium Chloride And Sodium Hydroxide Formula.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation ID2276630 Magnesium Chloride And Sodium Hydroxide Formula what is the formula for barium chloride? a novel process for obtaining magnesium from sea water involves several reactions. Barium is an alkaline earth and always corms a cation of charge of [+2],. Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. aqueous solutions of magnesium chloride. Magnesium Chloride And Sodium Hydroxide Formula.
From www.coursehero.com
[Solved] Magnesium chloride reacts with Sodium hydroxide. a) Write out Magnesium Chloride And Sodium Hydroxide Formula The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by. aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions. Magnesium Chloride And Sodium Hydroxide Formula.
From www.chegg.com
Solved Write the balanced chemical equation for the reaction Magnesium Chloride And Sodium Hydroxide Formula Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by. a novel process for obtaining magnesium from sea water involves several reactions. Barium is an alkaline earth and always corms a. Magnesium Chloride And Sodium Hydroxide Formula.
From mavink.com
Electron Dot Structure Of Sodium Chloride Magnesium Chloride And Sodium Hydroxide Formula a novel process for obtaining magnesium from sea water involves several reactions. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. Barium is an alkaline earth and always corms a cation of charge of [+2],. what is the formula for barium. Magnesium Chloride And Sodium Hydroxide Formula.
From tabithadesnhpotts.blogspot.com
Magnesium Chloride and Sodium Hydroxide Net Ionic Equation Magnesium Chloride And Sodium Hydroxide Formula Mgcl2 crystallizes in the cadmium chloride cdcl2 motif, which features octahedral mg centers. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide. Magnesium Chloride And Sodium Hydroxide Formula.
From exyfbegni.blob.core.windows.net
Magnesium And Zinc Chloride Balanced Equation at Jermaine Nixon blog Magnesium Chloride And Sodium Hydroxide Formula thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. what is the formula for barium chloride? a novel process for obtaining magnesium. Magnesium Chloride And Sodium Hydroxide Formula.
From www.chegg.com
Solved What is the full ionic equation for the reaction Magnesium Chloride And Sodium Hydroxide Formula Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. Mgcl2 crystallizes in the cadmium chloride cdcl2 motif, which features octahedral mg centers. Barium is an alkaline earth and always corms a cation of charge of [+2],. The reaction between methane and oxygen to yield carbon dioxide and water (shown at. Magnesium Chloride And Sodium Hydroxide Formula.
From www.chegg.com
Solved a Balance the reaction of sodium hydroxide and Magnesium Chloride And Sodium Hydroxide Formula thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. what is the formula for barium chloride? a novel process for obtaining magnesium. Magnesium Chloride And Sodium Hydroxide Formula.
From www.tessshebaylo.com
Balanced Chemical Equation For Sodium Sulfate And Water Tessshebaylo Magnesium Chloride And Sodium Hydroxide Formula aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different. Magnesium Chloride And Sodium Hydroxide Formula.
From www.thesciencehive.co.uk
Identifying Ions (AQA) — the science sauce Magnesium Chloride And Sodium Hydroxide Formula mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions are colourless. what is the formula for barium chloride? Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. Mgcl2 crystallizes in the cadmium chloride cdcl2 motif, which. Magnesium Chloride And Sodium Hydroxide Formula.
From www.youtube.com
How Sodium hydroxide (NaOH) react with Magnesium sulphate (MgSO4 Magnesium Chloride And Sodium Hydroxide Formula The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by. Mgcl2 crystallizes in the cadmium chloride cdcl2 motif, which features octahedral mg centers. what is the formula for barium chloride? Barium is an alkaline earth and always corms a cation of charge of [+2],. a novel process for obtaining. Magnesium Chloride And Sodium Hydroxide Formula.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Magnesium Chloride And Sodium Hydroxide Formula The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq). Magnesium Chloride And Sodium Hydroxide Formula.
From www.onlinemathlearning.com
Writing Ionic Equation (video lessons, examples and solutions) Magnesium Chloride And Sodium Hydroxide Formula aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. Mgcl2 crystallizes in the cadmium chloride cdcl2 motif, which features octahedral mg centers. a novel process for obtaining magnesium from sea water involves several reactions. Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where. Magnesium Chloride And Sodium Hydroxide Formula.
From www.numerade.com
SOLVEDWrite the net ionic equation for the reaction between solutions Magnesium Chloride And Sodium Hydroxide Formula a novel process for obtaining magnesium from sea water involves several reactions. aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions are colourless. Mgcl2 crystallizes in the. Magnesium Chloride And Sodium Hydroxide Formula.
From www.bartleby.com
Answered Magnesium hydroxide is insoluble. Write… bartleby Magnesium Chloride And Sodium Hydroxide Formula Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. Barium is an alkaline earth and always corms a cation of charge of [+2],. a novel process for obtaining magnesium from sea water involves several reactions. aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium. Magnesium Chloride And Sodium Hydroxide Formula.
From exyfbegni.blob.core.windows.net
Magnesium And Zinc Chloride Balanced Equation at Jermaine Nixon blog Magnesium Chloride And Sodium Hydroxide Formula Barium is an alkaline earth and always corms a cation of charge of [+2],. what is the formula for barium chloride? Mgcl2 crystallizes in the cadmium chloride cdcl2 motif, which features octahedral mg centers. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the. Magnesium Chloride And Sodium Hydroxide Formula.
From www.vrogue.co
Chemical Formula Of The Following Compounds By Crissc vrogue.co Magnesium Chloride And Sodium Hydroxide Formula a novel process for obtaining magnesium from sea water involves several reactions. The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by. Barium is an alkaline earth and always corms a cation of charge of [+2],. mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl. Magnesium Chloride And Sodium Hydroxide Formula.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Magnesium Chloride And Sodium Hydroxide Formula mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions are colourless. The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by. a novel process for obtaining magnesium from sea water involves several reactions. aqueous solutions of. Magnesium Chloride And Sodium Hydroxide Formula.
From www.youtube.com
How to Balance MgCl2 + NaHCO3 = MgCO3 + NaCl + H2O + CO2 Magnesium Magnesium Chloride And Sodium Hydroxide Formula a novel process for obtaining magnesium from sea water involves several reactions. The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom) may be represented by. aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. what is the formula for barium chloride? Mgcl2. Magnesium Chloride And Sodium Hydroxide Formula.
From www.sarthaks.com
Write down the formulae of these compounds, using criss cross method Magnesium Chloride And Sodium Hydroxide Formula mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions are colourless. a novel process for obtaining magnesium from sea water involves several reactions. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions. Magnesium Chloride And Sodium Hydroxide Formula.
From tabithadesnhpotts.blogspot.com
Magnesium Chloride and Sodium Hydroxide Net Ionic Equation Magnesium Chloride And Sodium Hydroxide Formula Barium is an alkaline earth and always corms a cation of charge of [+2],. aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. what is the formula for barium chloride? thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative. Magnesium Chloride And Sodium Hydroxide Formula.
From exyhaniao.blob.core.windows.net
G Magnesium Hydroxide Formula at Mose Newell blog Magnesium Chloride And Sodium Hydroxide Formula mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions are colourless. Mgcl2 crystallizes in the cadmium chloride cdcl2 motif, which features octahedral mg centers. a novel process for obtaining magnesium from sea water involves several reactions. what is the formula for barium chloride? Mgcl2 +. Magnesium Chloride And Sodium Hydroxide Formula.
From www.studypool.com
SOLUTION CHM1030 Columbia Southern Magnesium Chloride & Sodium Magnesium Chloride And Sodium Hydroxide Formula Mgcl2 crystallizes in the cadmium chloride cdcl2 motif, which features octahedral mg centers. Barium is an alkaline earth and always corms a cation of charge of [+2],. a novel process for obtaining magnesium from sea water involves several reactions. aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. . Magnesium Chloride And Sodium Hydroxide Formula.
From exyfbegni.blob.core.windows.net
Magnesium And Zinc Chloride Balanced Equation at Jermaine Nixon blog Magnesium Chloride And Sodium Hydroxide Formula what is the formula for barium chloride? mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions are colourless. Barium is an alkaline earth and always corms a cation of charge of [+2],. The reaction between methane and oxygen to yield carbon dioxide and water (shown at. Magnesium Chloride And Sodium Hydroxide Formula.
From www.numerade.com
SOLVED Aqueous solutions of magnesium chloride and sodium hydroxide Magnesium Chloride And Sodium Hydroxide Formula mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium hydroxide solutions are colourless. Barium is an alkaline earth and always corms a cation of charge of [+2],. Mgcl2 + naoh = mg (oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous. what is. Magnesium Chloride And Sodium Hydroxide Formula.
From www.numerade.com
SOLVED What will cause more Magnesium to be dissolved in a solution of Magnesium Chloride And Sodium Hydroxide Formula Barium is an alkaline earth and always corms a cation of charge of [+2],. aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. Mgcl2. Magnesium Chloride And Sodium Hydroxide Formula.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium Chloride And Sodium Hydroxide Formula aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. The reaction between methane and oxygen to yield carbon dioxide and water (shown at bottom). Magnesium Chloride And Sodium Hydroxide Formula.
From www.youtube.com
(a) Show the formation of magnesium chloride and sodium chloride by Magnesium Chloride And Sodium Hydroxide Formula thus, \(\ce{nacl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. Barium is an alkaline earth and always corms a cation of charge of [+2],. mgcl 2 (aq) + naoh (aq) = mg (oh) 2 (s) + nacl (aq) both magnesium nitrate and sodium. Magnesium Chloride And Sodium Hydroxide Formula.