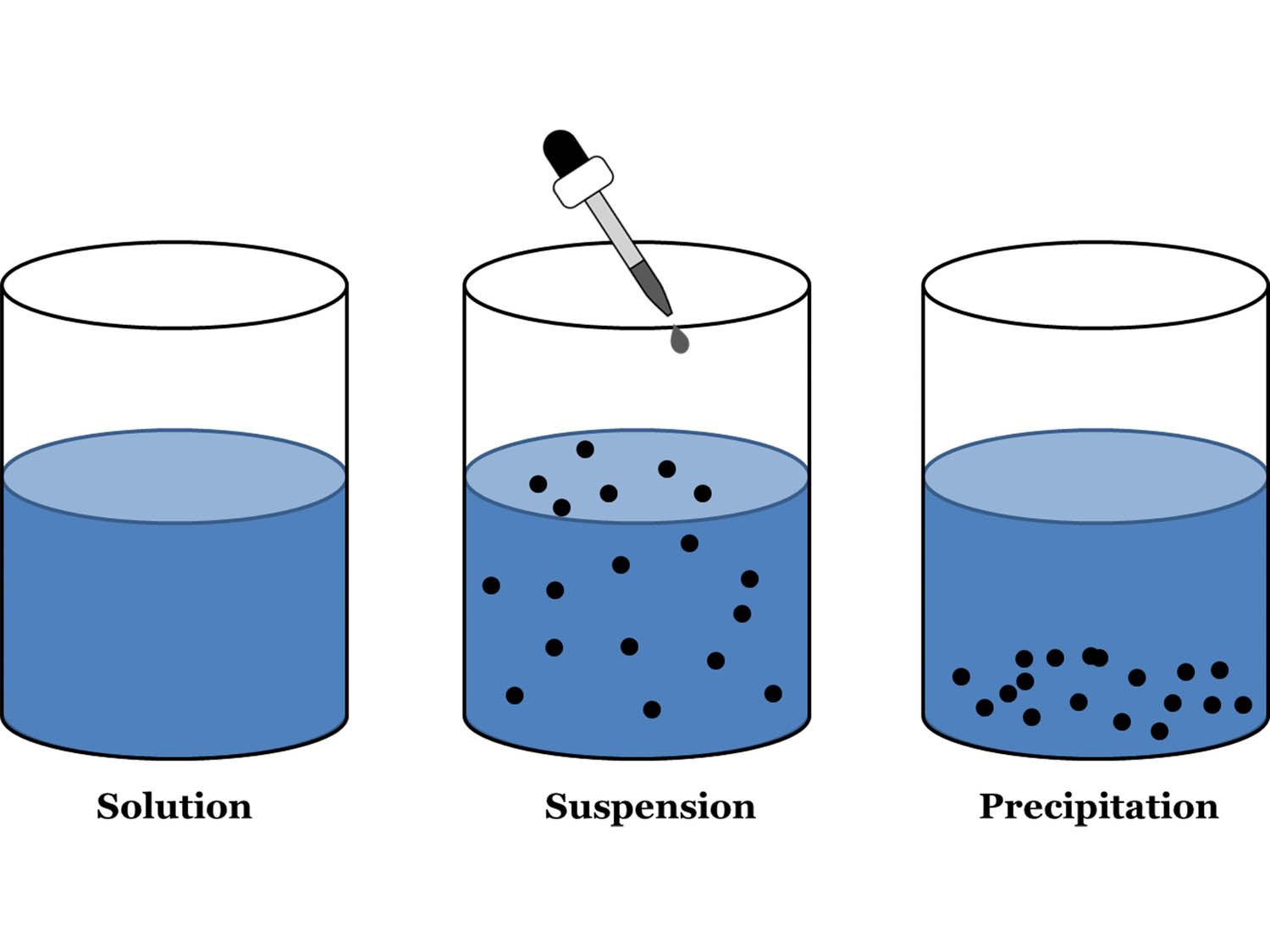
Difference Between Suspension And Colloid Class 9 . Sate three differences between suspensions and colloids. The main difference between colloid and suspension lies in the size of particles. Colloid particles are much smaller than suspension particles. It is a heterogeneous mixture in which solute particles do not dissolve but remain suspended, particles can be seen with naked eyes, it scatters a beam of light, particles can be. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. It can be separated by. Write three difference between colloidal solution, solution and suspension. Difference between solution, suspension and colloids. Suspension is a heterogeneous mixture of substance. Colloidal solution appears as a homogeneous mixture, but it also can exist.
from byjus.com
It can be separated by. Suspension is a heterogeneous mixture of substance. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. The main difference between colloid and suspension lies in the size of particles. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. Difference between solution, suspension and colloids. Colloidal solution appears as a homogeneous mixture, but it also can exist. Colloid particles are much smaller than suspension particles. Write three difference between colloidal solution, solution and suspension. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension.
Suspensions & Colloids Difference Between Colloid & SuspensionByju's
Difference Between Suspension And Colloid Class 9 Suspension is a heterogeneous mixture of substance. The main difference between colloid and suspension lies in the size of particles. Sate three differences between suspensions and colloids. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. Colloidal solution appears as a homogeneous mixture, but it also can exist. It can be separated by. Colloid particles are much smaller than suspension particles. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Suspension is a heterogeneous mixture of substance. It is a heterogeneous mixture in which solute particles do not dissolve but remain suspended, particles can be seen with naked eyes, it scatters a beam of light, particles can be. Write three difference between colloidal solution, solution and suspension. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Difference between solution, suspension and colloids.
From brainly.ph
im kinda struggling on science solutions, suspension, and colloid. Can Difference Between Suspension And Colloid Class 9 Sate three differences between suspensions and colloids. Difference between solution, suspension and colloids. Write three difference between colloidal solution, solution and suspension. The main difference between colloid and suspension lies in the size of particles. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. A colloid. Difference Between Suspension And Colloid Class 9.
From www.drrobertyoung.com
Understanding Colloids & Nano Colloidal Systems in Health & Nutrition Difference Between Suspension And Colloid Class 9 Colloidal solution appears as a homogeneous mixture, but it also can exist. Write three difference between colloidal solution, solution and suspension. Colloid particles are much smaller than suspension particles. Suspension is a heterogeneous mixture of substance. Difference between solution, suspension and colloids. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout. Difference Between Suspension And Colloid Class 9.
From www.studypage.in
True Solution, Colloidal Solution and Suspension Difference Between Suspension And Colloid Class 9 Write three difference between colloidal solution, solution and suspension. Colloidal solution appears as a homogeneous mixture, but it also can exist. Sate three differences between suspensions and colloids. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. Colloid particles are much smaller than suspension particles. The main difference between colloid and suspension lies in the. Difference Between Suspension And Colloid Class 9.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Difference Between Suspension And Colloid Class 9 A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Colloid particles are much smaller than suspension particles. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. Write three difference between colloidal solution, solution and suspension. Sate three differences between suspensions and colloids. It is. Difference Between Suspension And Colloid Class 9.
From www.pinterest.com
mixture suspension colloid Google Search Chemistry worksheets Difference Between Suspension And Colloid Class 9 It is a heterogeneous mixture in which solute particles do not dissolve but remain suspended, particles can be seen with naked eyes, it scatters a beam of light, particles can be. Sate three differences between suspensions and colloids. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Difference Between Suspension And Colloid Class 9.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Difference Between Suspension And Colloid Class 9 Colloid particles are much smaller than suspension particles. Write three difference between colloidal solution, solution and suspension. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Suspension is a heterogeneous mixture of substance. It is a heterogeneous mixture in which solute particles do not dissolve but. Difference Between Suspension And Colloid Class 9.
From www.youtube.com
Chemistry 9.4 Solutions, Colloids and Suspensions YouTube Difference Between Suspension And Colloid Class 9 A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Colloidal solution appears as a homogeneous mixture, but it also can exist. Difference between solution, suspension and colloids. Colloid particles are much smaller than suspension particles. Sate three differences between suspensions and colloids. It is a heterogeneous. Difference Between Suspension And Colloid Class 9.
From www.youtube.com
Solution colloids n suspension class 9 by neelam dwivedi YouTube Difference Between Suspension And Colloid Class 9 A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Write three difference between colloidal solution, solution and suspension. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. It can be separated by. Colloidal solution appears as a homogeneous mixture, but it. Difference Between Suspension And Colloid Class 9.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Difference Between Suspension And Colloid Class 9 It can be separated by. Colloid particles are much smaller than suspension particles. Colloidal solution appears as a homogeneous mixture, but it also can exist. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. Suspension is a heterogeneous mixture of substance. It is a heterogeneous mixture in which solute particles do not dissolve but remain. Difference Between Suspension And Colloid Class 9.
From mungfali.com
True Solution Colloid And Suspension Difference Between Suspension And Colloid Class 9 Write three difference between colloidal solution, solution and suspension. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. It is a heterogeneous mixture in which solute particles do not dissolve but remain suspended, particles can be seen with naked eyes, it scatters a beam of light, particles can be. Colloidal solution appears as a homogeneous. Difference Between Suspension And Colloid Class 9.
From brainly.in
Write three difference between solution and suspension and colloid Difference Between Suspension And Colloid Class 9 Sate three differences between suspensions and colloids. It is a heterogeneous mixture in which solute particles do not dissolve but remain suspended, particles can be seen with naked eyes, it scatters a beam of light, particles can be. Suspension is a heterogeneous mixture of substance. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. It. Difference Between Suspension And Colloid Class 9.
From edurev.in
Difference between true solution, suspension and colloidal sol Difference Between Suspension And Colloid Class 9 It is a heterogeneous mixture in which solute particles do not dissolve but remain suspended, particles can be seen with naked eyes, it scatters a beam of light, particles can be. Colloidal solution appears as a homogeneous mixture, but it also can exist. Write three difference between colloidal solution, solution and suspension. 1 nm − 100 nm (in diameter) larger. Difference Between Suspension And Colloid Class 9.
From www.youtube.com
Solution Suspension Colloid YouTube Difference Between Suspension And Colloid Class 9 Difference between solution, suspension and colloids. Sate three differences between suspensions and colloids. Colloid particles are much smaller than suspension particles. The main difference between colloid and suspension lies in the size of particles. It is a heterogeneous mixture in which solute particles do not dissolve but remain suspended, particles can be seen with naked eyes, it scatters a beam. Difference Between Suspension And Colloid Class 9.
From www.youtube.com
Science 9 Chapter 2 Suspension, Colloid, Tyndall effect, Comparison of Difference Between Suspension And Colloid Class 9 Colloidal solution appears as a homogeneous mixture, but it also can exist. Suspension is a heterogeneous mixture of substance. Colloid particles are much smaller than suspension particles. Difference between solution, suspension and colloids. It can be separated by. Sate three differences between suspensions and colloids. A colloid is a mixture in which one of the soluble or insoluble particles is. Difference Between Suspension And Colloid Class 9.
From www.thoughtco.com
Tyndall Effect Definition and Examples Difference Between Suspension And Colloid Class 9 A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Sate three differences between suspensions and colloids. Difference between solution, suspension and colloids. Colloid particles are much smaller than suspension particles. Suspension is a heterogeneous mixture of substance. It is a heterogeneous mixture in which solute particles. Difference Between Suspension And Colloid Class 9.
From www.toppr.com
(k) Differentiate between solution, suspension and colloids on the Difference Between Suspension And Colloid Class 9 The main difference between colloid and suspension lies in the size of particles. It can be separated by. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the. Difference Between Suspension And Colloid Class 9.
From www.differencebetween.com
Difference Between Suspension and Colloid Compare the Difference Difference Between Suspension And Colloid Class 9 Sate three differences between suspensions and colloids. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. It is a heterogeneous mixture in which solute particles do not dissolve but remain suspended, particles can be seen. Difference Between Suspension And Colloid Class 9.
From brainly.in
How are colloids and suspensions different from solutions ? Brainly.in Difference Between Suspension And Colloid Class 9 Write three difference between colloidal solution, solution and suspension. Colloidal solution appears as a homogeneous mixture, but it also can exist. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. It is a heterogeneous mixture in which solute particles do not dissolve but remain suspended, particles. Difference Between Suspension And Colloid Class 9.
From gioglbtpv.blob.core.windows.net
Suspension And Colloid And Solution at Jason Vasquez blog Difference Between Suspension And Colloid Class 9 A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Write three difference between colloidal solution, solution and suspension. It can be separated by. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. A colloid is a mixture in which one of. Difference Between Suspension And Colloid Class 9.
From www.sliderbase.com
Colloids and Suspensions Presentation Chemistry Difference Between Suspension And Colloid Class 9 It can be separated by. Write three difference between colloidal solution, solution and suspension. Colloidal solution appears as a homogeneous mixture, but it also can exist. Difference between solution, suspension and colloids. It is a heterogeneous mixture in which solute particles do not dissolve but remain suspended, particles can be seen with naked eyes, it scatters a beam of light,. Difference Between Suspension And Colloid Class 9.
From www.youtube.com
TRUE SOLUTION COLLOID SUSPENSIONS 10 major differences. YouTube Difference Between Suspension And Colloid Class 9 It can be separated by. Difference between solution, suspension and colloids. Suspension is a heterogeneous mixture of substance. Write three difference between colloidal solution, solution and suspension. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Sate three differences between suspensions and colloids. A colloid is. Difference Between Suspension And Colloid Class 9.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube Difference Between Suspension And Colloid Class 9 It can be separated by. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. Sate three differences between suspensions and colloids. The main difference between colloid and suspension lies in the size of particles. Colloidal. Difference Between Suspension And Colloid Class 9.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram Difference Between Suspension And Colloid Class 9 The main difference between colloid and suspension lies in the size of particles. Colloid particles are much smaller than suspension particles. Difference between solution, suspension and colloids. Suspension is a heterogeneous mixture of substance. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. 1 nm − 100 nm (in. Difference Between Suspension And Colloid Class 9.
From animalia-life.club
Properties Of Colloids Tyndall Effect Difference Between Suspension And Colloid Class 9 Suspension is a heterogeneous mixture of substance. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. The main difference between colloid and suspension lies in the size of particles. Colloid particles are much smaller than suspension particles. Sate three differences between suspensions and colloids. Write three difference between colloidal solution, solution and suspension. A colloid. Difference Between Suspension And Colloid Class 9.
From edurev.in
what is colloid.solutions EduRev Class 9 Question Difference Between Suspension And Colloid Class 9 It can be separated by. Colloid particles are much smaller than suspension particles. Write three difference between colloidal solution, solution and suspension. Colloidal solution appears as a homogeneous mixture, but it also can exist. Difference between solution, suspension and colloids. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other.. Difference Between Suspension And Colloid Class 9.
From brainly.in
draw diagram illustrate true solutions suspension and colloidal Difference Between Suspension And Colloid Class 9 Difference between solution, suspension and colloids. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Colloid particles are much smaller than suspension particles. Sate three differences between suspensions and colloids. It can be separated by. Suspension is a heterogeneous mixture of substance. Write three difference between colloidal solution, solution. Difference Between Suspension And Colloid Class 9.
From gionidedc.blob.core.windows.net
Colloid Solution In at Martin Miller blog Difference Between Suspension And Colloid Class 9 Difference between solution, suspension and colloids. The main difference between colloid and suspension lies in the size of particles. Colloidal solution appears as a homogeneous mixture, but it also can exist. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Suspension is a heterogeneous mixture of. Difference Between Suspension And Colloid Class 9.
From byjus.com
What is difference between true solution and suspension and colloid? Difference Between Suspension And Colloid Class 9 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. Write three difference between colloidal solution, solution and suspension. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. It can be separated by. Difference between solution, suspension and colloids. Colloid particles are much smaller than. Difference Between Suspension And Colloid Class 9.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube Difference Between Suspension And Colloid Class 9 Colloid particles are much smaller than suspension particles. Sate three differences between suspensions and colloids. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. The main difference between colloid and suspension lies in the size of particles. Write three difference between colloidal solution, solution and suspension. A colloid is a heterogeneous mixture in which the. Difference Between Suspension And Colloid Class 9.
From pediaa.com
Difference Between Lysosome and Peroxisome Characteristics, Structure Difference Between Suspension And Colloid Class 9 Colloidal solution appears as a homogeneous mixture, but it also can exist. Colloid particles are much smaller than suspension particles. It can be separated by. The main difference between colloid and suspension lies in the size of particles. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. It is. Difference Between Suspension And Colloid Class 9.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Difference Between Suspension And Colloid Class 9 A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. It can be separated by. Colloid particles are much smaller than suspension particles. Write three difference between colloidal solution, solution and suspension. It is a heterogeneous mixture in which solute particles do not dissolve but remain suspended, particles can be. Difference Between Suspension And Colloid Class 9.
From www.youtube.com
Solutions Suspensions and Colloids Part 1/1 English Class 9 YouTube Difference Between Suspension And Colloid Class 9 Colloidal solution appears as a homogeneous mixture, but it also can exist. Suspension is a heterogeneous mixture of substance. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. Write three difference between. Difference Between Suspension And Colloid Class 9.
From www.meritnation.com
Distinguish between true solution , suspension and colloid in a tabular Difference Between Suspension And Colloid Class 9 Write three difference between colloidal solution, solution and suspension. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. 1 nm − 100 nm (in diameter) larger than 100 nm (in diameter) separation. It can be separated by. Sate three differences between suspensions and colloids. A colloid. Difference Between Suspension And Colloid Class 9.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Difference Between Suspension And Colloid Class 9 Suspension is a heterogeneous mixture of substance. The main difference between colloid and suspension lies in the size of particles. It is a heterogeneous mixture in which solute particles do not dissolve but remain suspended, particles can be seen with naked eyes, it scatters a beam of light, particles can be. Write three difference between colloidal solution, solution and suspension.. Difference Between Suspension And Colloid Class 9.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Difference Between Suspension And Colloid Class 9 A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Colloidal solution appears as a homogeneous mixture, but it also can exist. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. 1 nm − 100 nm. Difference Between Suspension And Colloid Class 9.