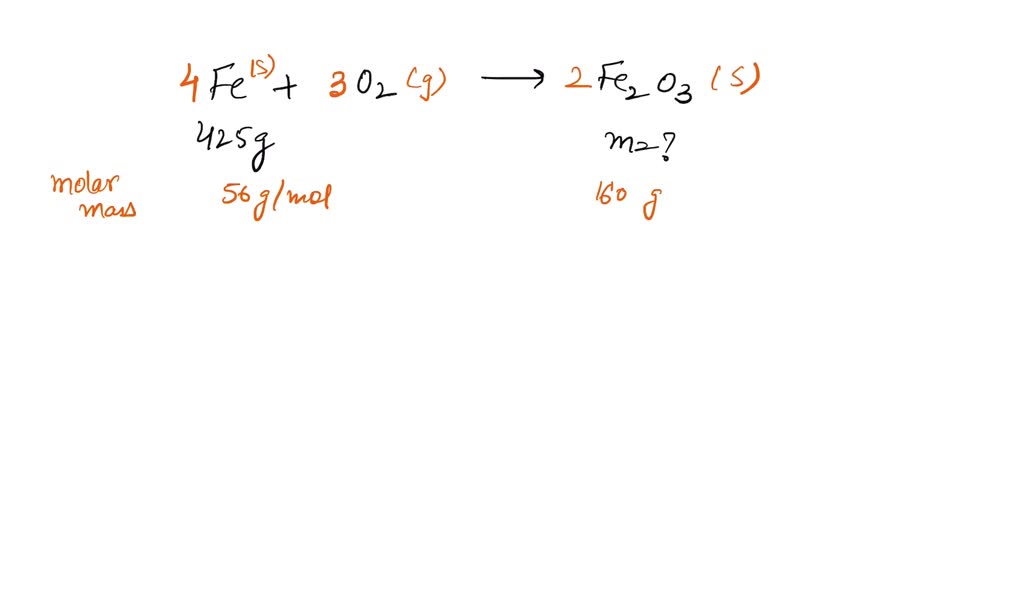Iron Iii Oxide Skeleton Equation . The chemical equation for this reaction is: Oxidation is the process in which an atom loses an electron. The oxidation state of fe 2 o 3 is +3. Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. It occurs in nature as the mineral hematite , which serves as the primary source of iron for the steel. Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe 2 o 3. The reactants in this reaction are iron(ii) oxide (feo) and oxygen (o2), and the product is. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. It is formed when iron undergoes oxidation.
from www.numerade.com
Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; It is formed when iron undergoes oxidation. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. The chemical equation for this reaction is: Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe 2 o 3. The oxidation state of fe 2 o 3 is +3. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms.
SOLVED of iron with oxygen gas and water produces iron (III) oxide (a
Iron Iii Oxide Skeleton Equation The reactants in this reaction are iron(ii) oxide (feo) and oxygen (o2), and the product is. Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; The chemical equation for this reaction is: Oxidation is the process in which an atom loses an electron. The reactants in this reaction are iron(ii) oxide (feo) and oxygen (o2), and the product is. Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe 2 o 3. The oxidation state of fe 2 o 3 is +3. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. It is formed when iron undergoes oxidation. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. It occurs in nature as the mineral hematite , which serves as the primary source of iron for the steel. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID2435173 Iron Iii Oxide Skeleton Equation Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. The reactants in this reaction are iron(ii) oxide (feo) and oxygen (o2), and the product is. Oxidation is the process. Iron Iii Oxide Skeleton Equation.
From www.numerade.com
SOLVED The thermite reaction involves aluminum and iron(III) oxide Iron Iii Oxide Skeleton Equation Oxidation is the process in which an atom loses an electron. It occurs in nature as the mineral hematite , which serves as the primary source of iron for the steel. The chemical equation for this reaction is: 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. The oxidation state of fe 2 o 3 is +3. Iron(ii) oxide reacts with oxygen to. Iron Iii Oxide Skeleton Equation.
From www.toppr.com
Iron III Oxide Formula Definition, Concepts and Examples Iron Iii Oxide Skeleton Equation The chemical equation for this reaction is: Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe 2 o 3. Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. The reactants in this reaction are iron(ii) oxide (feo) and oxygen (o2), and the product is. It occurs in nature as the mineral hematite , which serves as. Iron Iii Oxide Skeleton Equation.
From www.youtube.com
when iron rusts, solid iron reacts with gaseous oxygen to form solid Iron Iii Oxide Skeleton Equation The oxidation state of fe 2 o 3 is +3. Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe 2 o 3. The reactants in this reaction are iron(ii) oxide (feo) and oxygen (o2), and the product is. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. 2𝐹𝑒 (𝑂𝐻)₃. Iron Iii Oxide Skeleton Equation.
From www.youtube.com
Lewis Structure of Fe2O3, Iron (III) Oxide YouTube Iron Iii Oxide Skeleton Equation The reactants in this reaction are iron(ii) oxide (feo) and oxygen (o2), and the product is. Oxidation is the process in which an atom loses an electron. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. The chemical equation for this reaction is: Fe 2 o 3 is the chemical formula of iron(iii). Iron Iii Oxide Skeleton Equation.
From www.numerade.com
SOLVED of iron with oxygen gas and water produces iron (III) oxide (a Iron Iii Oxide Skeleton Equation Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. Oxidation is the process in which an atom loses an electron. It is formed when iron undergoes oxidation. The chemical equation for this reaction is: Iron(iii) oxide reacts with chlorine gas. Iron Iii Oxide Skeleton Equation.
From www.showme.com
Write the formula for iron (III) oxide and iron (III) sulfate Iron Iii Oxide Skeleton Equation The reactants in this reaction are iron(ii) oxide (feo) and oxygen (o2), and the product is. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ +. Iron Iii Oxide Skeleton Equation.
From testbook.com
Iron(III) Oxide Formula Know Its Preparation, Properties & Uses Iron Iii Oxide Skeleton Equation It occurs in nature as the mineral hematite , which serves as the primary source of iron for the steel. The oxidation state of fe 2 o 3 is +3. Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. The chemical equation for this reaction is: Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according. Iron Iii Oxide Skeleton Equation.
From www.numerade.com
SOLVED Calculate the mass of Iron(III) oxide (FezO;) that contains a Iron Iii Oxide Skeleton Equation Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; The chemical equation for this reaction is: Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. The oxidation state of fe 2 o 3 is. Iron Iii Oxide Skeleton Equation.
From www.coursehero.com
[Solved] In the following reaction, if 65.2 g of Iron (III) oxide Iron Iii Oxide Skeleton Equation Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; It is formed when iron. Iron Iii Oxide Skeleton Equation.
From www.coursehero.com
[Solved] Iron (III) oxide is formed when iron combines with oxygen in Iron Iii Oxide Skeleton Equation The chemical equation for this reaction is: The oxidation state of fe 2 o 3 is +3. Oxidation is the process in which an atom loses an electron. It is formed when iron undergoes oxidation. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. Iron(ii) oxide reacts with oxygen to form iron(iii) oxide.. Iron Iii Oxide Skeleton Equation.
From www.chegg.com
Solved 3. Iron reacts with oxygen to form iron (III) oxide Iron Iii Oxide Skeleton Equation Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. The reactants in this reaction are iron(ii) oxide (feo) and oxygen (o2), and. Iron Iii Oxide Skeleton Equation.
From www.youtube.com
How to Write the Formula for Iron (III) Oxide YouTube Iron Iii Oxide Skeleton Equation Oxidation is the process in which an atom loses an electron. The chemical equation for this reaction is: Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; The oxidation state of fe 2 o 3 is +3. Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe. Iron Iii Oxide Skeleton Equation.
From www.numerade.com
SOLVEDWrite skeleton equations for these reactio… Iron Iii Oxide Skeleton Equation 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. It occurs in nature as the mineral hematite , which serves as the primary source of iron for the steel. Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; It is formed when iron undergoes oxidation. Oxidation is the process in which an atom. Iron Iii Oxide Skeleton Equation.
From www.showme.com
Iron III oxide Science, Chemistry, Chemical Bonds ShowMe Iron Iii Oxide Skeleton Equation It occurs in nature as the mineral hematite , which serves as the primary source of iron for the steel. Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Oxidation is the process in which an atom loses an electron. Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe 2. Iron Iii Oxide Skeleton Equation.
From www.researchgate.net
Figure ED 5.10.51 Homological series of iron oxides described by the Iron Iii Oxide Skeleton Equation 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe 2 o 3. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. The oxidation state of fe 2 o 3 is +3. The chemical equation for this reaction is: Fe 2 o 3 is the chemical formula of iron(iii). Iron Iii Oxide Skeleton Equation.
From www.numerade.com
SOLVED Question 2 10 pts In a redox reaction, solid iron (III) oxide Iron Iii Oxide Skeleton Equation Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. The reactants in this reaction are iron(ii) oxide (feo) and oxygen (o2), and the product is. Both methods result in. Iron Iii Oxide Skeleton Equation.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Iron Iii Oxide Skeleton Equation Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe 2 o 3. It occurs in nature as the mineral hematite , which serves as the primary source of iron for the steel. It is formed when iron undergoes oxidation. Oxidation is the process in which an atom loses an electron. The chemical equation for this reaction. Iron Iii Oxide Skeleton Equation.
From www.pw.live
Iron III Oxide Formula, Structure, Properties, Uses Iron Iii Oxide Skeleton Equation Oxidation is the process in which an atom loses an electron. Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe 2 o 3. The chemical equation for this reaction is: It is formed when iron undergoes oxidation. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron. Iron Iii Oxide Skeleton Equation.
From www.youtube.com
Mass of iron, Fe, formed from iron oxide, Fe2O3, moles calculation Iron Iii Oxide Skeleton Equation 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. It is formed when iron undergoes oxidation. The chemical equation for this reaction is: The oxidation state of fe 2 o 3 is +3. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according. Iron Iii Oxide Skeleton Equation.
From stock.adobe.com
Iron Iii Oxide Properties and Chemical Compound Structure Stock Vector Iron Iii Oxide Skeleton Equation The oxidation state of fe 2 o 3 is +3. The chemical equation for this reaction is: Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Oxidation is the process in which an atom loses an electron. 2𝐹𝑒 (𝑂𝐻)₃. Iron Iii Oxide Skeleton Equation.
From www.slideserve.com
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation ID6658952 Iron Iii Oxide Skeleton Equation The oxidation state of fe 2 o 3 is +3. The chemical equation for this reaction is: Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Oxidation is the process in which an atom loses an electron. Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. It is formed when iron undergoes oxidation. Iron(iii) oxide or ferric oxide. Iron Iii Oxide Skeleton Equation.
From www.vrogue.co
What Is Formed In The Reaction Between Iron And Oxyge vrogue.co Iron Iii Oxide Skeleton Equation Oxidation is the process in which an atom loses an electron. The oxidation state of fe 2 o 3 is +3. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. It is formed when iron undergoes. Iron Iii Oxide Skeleton Equation.
From www.numerade.com
SOLVEDIron metal reacts with oxygen to give iron(III) oxide, Fe2 O3 (a Iron Iii Oxide Skeleton Equation Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. It occurs in nature as the mineral hematite , which serves as the primary source of iron for. Iron Iii Oxide Skeleton Equation.
From www.youtube.com
Al+Fe2O3=Al2O3+Fe Balanced EquationAluminum+Iron(III) oxide+Iron Iron Iii Oxide Skeleton Equation Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. The reactants in this reaction are iron(ii) oxide (feo) and oxygen (o2), and the product is. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. Iron(iii) oxide or ferric oxide is the inorganic. Iron Iii Oxide Skeleton Equation.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation ID3552860 Iron Iii Oxide Skeleton Equation Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; The oxidation state of fe 2 o 3 is +3. The reactants in this reaction are iron(ii) oxide (feo) and oxygen (o2), and the product is. It occurs in nature as the. Iron Iii Oxide Skeleton Equation.
From www.answersarena.com
[Solved] Please refer to image attached Iron(III) oxide re Iron Iii Oxide Skeleton Equation 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. The chemical equation for this reaction is: Oxidation is the process in which an atom loses an electron. Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe 2 o 3. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. It occurs in nature. Iron Iii Oxide Skeleton Equation.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation ID6634764 Iron Iii Oxide Skeleton Equation Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. It is formed when iron undergoes oxidation. Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe 2 o 3. The oxidation state of fe 2 o 3 is +3. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. The. Iron Iii Oxide Skeleton Equation.
From www.numerade.com
SOLVED Iron (III) oxide reacts with carbon monoxide to produce iron Iron Iii Oxide Skeleton Equation Iron (iii) oxide has the chemical formula {eq}fe_ {2}o_ {3} {/eq}. The chemical equation for this reaction is: It is formed when iron undergoes oxidation. Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. Oxidation is the process in which. Iron Iii Oxide Skeleton Equation.
From www.numerade.com
SOLVED Iron reacts with oxygen to form iron(III) oxide according to Iron Iii Oxide Skeleton Equation Oxidation is the process in which an atom loses an electron. Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe 2 o 3. Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. It is formed when iron undergoes oxidation. The. Iron Iii Oxide Skeleton Equation.
From www.numerade.com
SOLVED Write the skeleton equation for each of the following reactions Iron Iii Oxide Skeleton Equation Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. It occurs in nature as the mineral hematite , which serves as the primary source of iron for the steel. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including. Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to. Iron Iii Oxide Skeleton Equation.
From www.youtube.com
How to write the formula for iron (III) oxide YouTube Iron Iii Oxide Skeleton Equation Oxidation is the process in which an atom loses an electron. It is formed when iron undergoes oxidation. The oxidation state of fe 2 o 3 is +3. Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; The chemical equation for this reaction is: 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂.. Iron Iii Oxide Skeleton Equation.
From www.coursehero.com
[Solved] In the following reaction, if 65.2 g of Iron (III) oxide Iron Iii Oxide Skeleton Equation The reactants in this reaction are iron(ii) oxide (feo) and oxygen (o2), and the product is. It is formed when iron undergoes oxidation. It occurs in nature as the mineral hematite , which serves as the primary source of iron for the steel. The oxidation state of fe 2 o 3 is +3. Both methods result in the formation of. Iron Iii Oxide Skeleton Equation.
From brainly.com
Calculator the mass of iron (III) oxide ( Fe2O3) that contains a Iron Iii Oxide Skeleton Equation Iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe 2 o 3. The chemical equation for this reaction is: Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. The oxidation. Iron Iii Oxide Skeleton Equation.
From www.slideserve.com
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379 Iron Iii Oxide Skeleton Equation Iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; Oxidation is the process in which an atom loses an electron. It occurs in nature as the mineral hematite , which serves as the primary source of iron for the steel. Iron(ii) oxide reacts with oxygen to form iron(iii) oxide. 2𝐹𝑒. Iron Iii Oxide Skeleton Equation.