Is Hf Stronger Than Hcn . As the concentration of hydrofluoric acid approaches 100 percent, it's acidity increases because of homoassociation, where a base and conjugate acid form a bond: acid and base chart lists the strength of acids and bases (strongest to weakest) in order. The strong bases are listed at the bottom right of the table and. determine the ionization constant of \(\ce{nh4+}\), and decide which is the stronger acid, hcn or \(\ce{nh4+}\). 33 rows acid with values less than one are considered weak. of these acids the strongest is hf as it has the largest k a. Hydrocyanic is the weakest of the group as it has the smallest k a. hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. determine the ionization constant of [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex], and decide which is the stronger acid, hcn or [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex]. the enthalpy change for hf is much smaller in magnitude than that for the other three hydrogen halides, but it is still. for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. Simple to use laboratory reference chart for scientists,.
from www.sarthaks.com
Hydrocyanic is the weakest of the group as it has the smallest k a. acid and base chart lists the strength of acids and bases (strongest to weakest) in order. As the concentration of hydrofluoric acid approaches 100 percent, it's acidity increases because of homoassociation, where a base and conjugate acid form a bond: of these acids the strongest is hf as it has the largest k a. hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. Simple to use laboratory reference chart for scientists,. the enthalpy change for hf is much smaller in magnitude than that for the other three hydrogen halides, but it is still. determine the ionization constant of \(\ce{nh4+}\), and decide which is the stronger acid, hcn or \(\ce{nh4+}\). determine the ionization constant of [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex], and decide which is the stronger acid, hcn or [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex]. 33 rows acid with values less than one are considered weak.
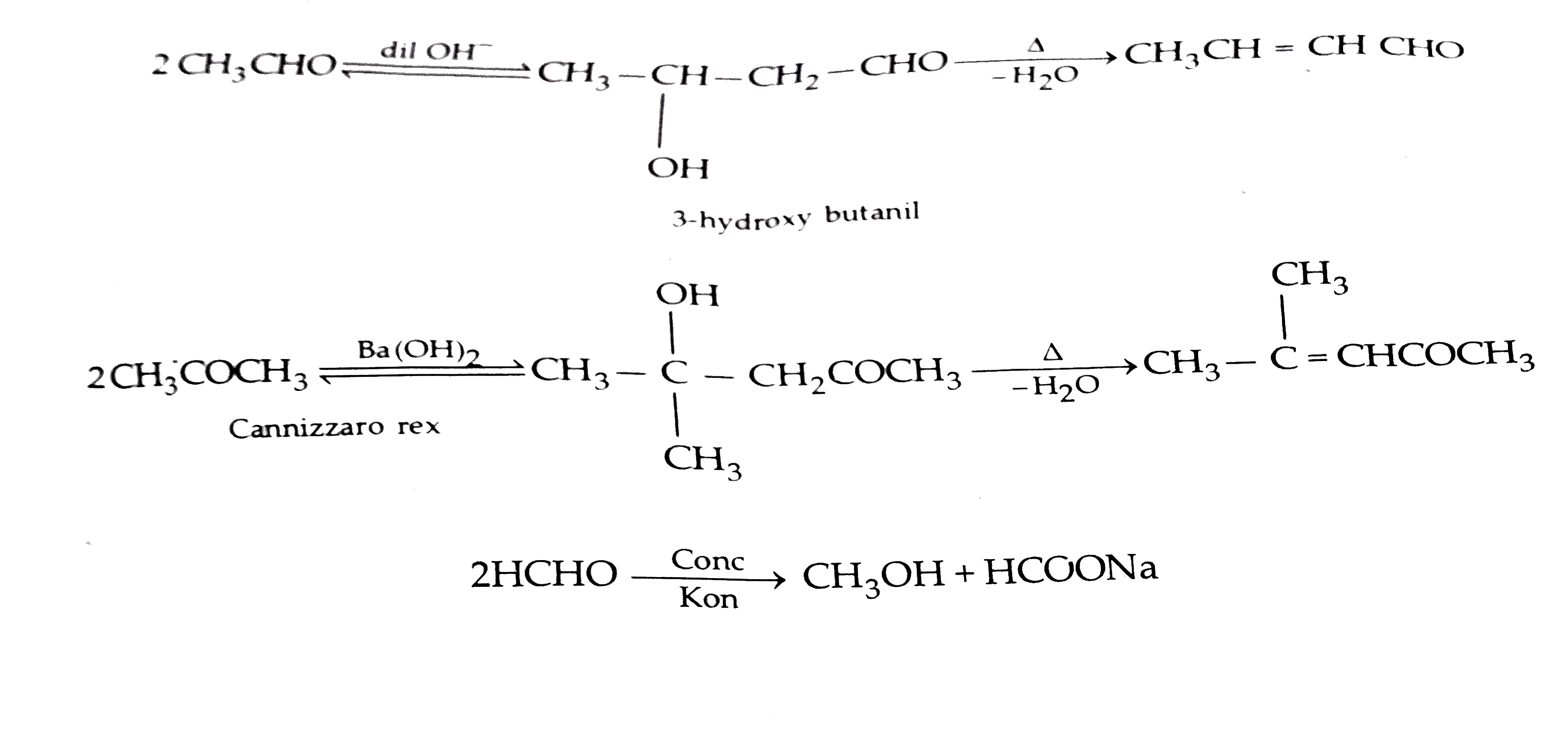
Account for the following (i) `CH_(3)CHO` is more reactive than `CH
Is Hf Stronger Than Hcn The strong bases are listed at the bottom right of the table and. the enthalpy change for hf is much smaller in magnitude than that for the other three hydrogen halides, but it is still. Simple to use laboratory reference chart for scientists,. As the concentration of hydrofluoric acid approaches 100 percent, it's acidity increases because of homoassociation, where a base and conjugate acid form a bond: The strong bases are listed at the bottom right of the table and. 33 rows acid with values less than one are considered weak. determine the ionization constant of \(\ce{nh4+}\), and decide which is the stronger acid, hcn or \(\ce{nh4+}\). determine the ionization constant of [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex], and decide which is the stronger acid, hcn or [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex]. of these acids the strongest is hf as it has the largest k a. hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. acid and base chart lists the strength of acids and bases (strongest to weakest) in order. for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. Hydrocyanic is the weakest of the group as it has the smallest k a.
From answers.yahoo.com
What does HCN look like? Yahoo Answers Is Hf Stronger Than Hcn for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. determine the ionization constant of [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex], and decide which is the stronger acid, hcn or [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex]. 33 rows acid with values less than one are considered weak. Hydrocyanic is the weakest of the group as it has the smallest. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVED[Rexien Toplcs] [Referoncos] Use the Relerences ACCEpOrtant Is Hf Stronger Than Hcn Hydrocyanic is the weakest of the group as it has the smallest k a. determine the ionization constant of [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex], and decide which is the stronger acid, hcn or [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex]. hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. determine the ionization constant of \(\ce{nh4+}\), and decide which is the. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVED Which of the following statements is true? HCIO4 is stronger Is Hf Stronger Than Hcn of these acids the strongest is hf as it has the largest k a. the enthalpy change for hf is much smaller in magnitude than that for the other three hydrogen halides, but it is still. acid and base chart lists the strength of acids and bases (strongest to weakest) in order. As the concentration of hydrofluoric. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVED Question 24 (2 points) HCI is a stronger acid than HF because Is Hf Stronger Than Hcn acid and base chart lists the strength of acids and bases (strongest to weakest) in order. 33 rows acid with values less than one are considered weak. for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. hydrofluoric acid is a much stronger acid when it is concentrated. Is Hf Stronger Than Hcn.
From ar.inspiredpencil.com
Intermolecular Forces Hf Is Hf Stronger Than Hcn Simple to use laboratory reference chart for scientists,. hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. As the concentration of hydrofluoric acid approaches 100 percent, it's acidity increases because of homoassociation, where. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVED Given the following information hydrocyanic acid nitrous acid Is Hf Stronger Than Hcn for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. As the concentration of hydrofluoric acid approaches 100 percent, it's acidity increases because of homoassociation, where a base and conjugate acid form a bond: determine the ionization constant of \(\ce{nh4+}\), and decide which is the stronger acid, hcn or \(\ce{nh4+}\).. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVED HF is a stronger acid than HCN. It will have (more/ less Is Hf Stronger Than Hcn for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. determine the ionization constant of \(\ce{nh4+}\), and decide which is the stronger acid, hcn or \(\ce{nh4+}\). Hydrocyanic is the weakest of the group as it has the smallest k a. As the concentration of hydrofluoric acid approaches 100 percent, it's. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVED Hydrobromic acid (HBr) is strong acid. Chlorous acid (HCIO ) is Is Hf Stronger Than Hcn for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. Hydrocyanic is the weakest of the group as it has the smallest k a. Simple to use laboratory reference chart for scientists,. the enthalpy change for hf is much smaller in magnitude than that for the other three hydrogen halides,. Is Hf Stronger Than Hcn.
From www.youtube.com
Why water has stronger hydrogen bond than HF and NH3 ? YouTube Is Hf Stronger Than Hcn the enthalpy change for hf is much smaller in magnitude than that for the other three hydrogen halides, but it is still. As the concentration of hydrofluoric acid approaches 100 percent, it's acidity increases because of homoassociation, where a base and conjugate acid form a bond: hydrofluoric acid is a much stronger acid when it is concentrated than. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVED HF is a stronger acid than HCN. Which of the following Is Hf Stronger Than Hcn acid and base chart lists the strength of acids and bases (strongest to weakest) in order. Hydrocyanic is the weakest of the group as it has the smallest k a. 33 rows acid with values less than one are considered weak. for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger. Is Hf Stronger Than Hcn.
From www.chegg.com
Solved Consider separate solutions of HF and HCN, both 0.35M Is Hf Stronger Than Hcn hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. As the concentration of hydrofluoric acid approaches 100 percent, it's acidity increases because of homoassociation, where a base and conjugate acid form a bond: 33 rows acid with values less than one are considered weak. of these acids the strongest is. Is Hf Stronger Than Hcn.
From www.sarthaks.com
Account for the following (i) `CH_(3)CHO` is more reactive than `CH Is Hf Stronger Than Hcn acid and base chart lists the strength of acids and bases (strongest to weakest) in order. Simple to use laboratory reference chart for scientists,. 33 rows acid with values less than one are considered weak. the enthalpy change for hf is much smaller in magnitude than that for the other three hydrogen halides, but it is still.. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVEDPredict whether the equilibrium lies to the right or to the left Is Hf Stronger Than Hcn acid and base chart lists the strength of acids and bases (strongest to weakest) in order. of these acids the strongest is hf as it has the largest k a. hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. determine the ionization constant of \(\ce{nh4+}\), and decide which is. Is Hf Stronger Than Hcn.
From www.slideserve.com
PPT Chapter 15 Acids and Bases PowerPoint Presentation, free download Is Hf Stronger Than Hcn hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. the enthalpy change for hf is much smaller in magnitude than that for the other three hydrogen halides, but it is still. determine the ionization constant of \(\ce{nh4+}\), and decide which is the stronger acid, hcn or \(\ce{nh4+}\). of these. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVED Given the following information hydrocyanic acid = HCN acetic Is Hf Stronger Than Hcn The strong bases are listed at the bottom right of the table and. of these acids the strongest is hf as it has the largest k a. the enthalpy change for hf is much smaller in magnitude than that for the other three hydrogen halides, but it is still. for example, nitrous acid (\(hno_2\)), with a \(pk_a\). Is Hf Stronger Than Hcn.
From www.coursehero.com
[Solved] HCI is a stronger acid than HF because The HCI bond is Is Hf Stronger Than Hcn Simple to use laboratory reference chart for scientists,. determine the ionization constant of \(\ce{nh4+}\), and decide which is the stronger acid, hcn or \(\ce{nh4+}\). acid and base chart lists the strength of acids and bases (strongest to weakest) in order. for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid. Is Hf Stronger Than Hcn.
From byjus.com
Account for the following (1) CH3CHO is more reactive with HCN than Is Hf Stronger Than Hcn the enthalpy change for hf is much smaller in magnitude than that for the other three hydrogen halides, but it is still. Simple to use laboratory reference chart for scientists,. determine the ionization constant of \(\ce{nh4+}\), and decide which is the stronger acid, hcn or \(\ce{nh4+}\). of these acids the strongest is hf as it has the. Is Hf Stronger Than Hcn.
From www.chegg.com
Solved Question 1 HF is a acid than HCl because stronger, F Is Hf Stronger Than Hcn 33 rows acid with values less than one are considered weak. for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. Hydrocyanic is the weakest of the group as it has the smallest k a. acid and base chart lists the strength of acids and bases (strongest to weakest). Is Hf Stronger Than Hcn.
From www.chegg.com
Solved Given the following information hydrocyanic acid HCN Is Hf Stronger Than Hcn for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. The strong bases are listed at the bottom right of the table and. determine the ionization constant of \(\ce{nh4+}\), and decide which is the stronger acid, hcn or \(\ce{nh4+}\). the enthalpy change for hf is much smaller in magnitude. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVED HCI (aq) is a strong acid, which means that HCl is a stronger Is Hf Stronger Than Hcn determine the ionization constant of [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex], and decide which is the stronger acid, hcn or [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex]. of these acids the strongest is hf as it has the largest k a. hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. Simple to use laboratory reference chart for scientists,. the enthalpy. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVED Consider the following equilibrium HF(aq) + CN(aq) ⇌ HCN(aq Is Hf Stronger Than Hcn for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. of these acids the strongest is hf as it has the largest k a. determine the ionization constant of [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex], and decide which is the stronger acid, hcn or [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex]. 33 rows acid with values less than one. Is Hf Stronger Than Hcn.
From techiescientist.com
HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and Is Hf Stronger Than Hcn Hydrocyanic is the weakest of the group as it has the smallest k a. determine the ionization constant of [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex], and decide which is the stronger acid, hcn or [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex]. The strong bases are listed at the bottom right of the table and. for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times. Is Hf Stronger Than Hcn.
From www.slideserve.com
PPT Ch.13 ions in aqueous solutions and colligative properties Is Hf Stronger Than Hcn As the concentration of hydrofluoric acid approaches 100 percent, it's acidity increases because of homoassociation, where a base and conjugate acid form a bond: hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. 33 rows acid with values less than one are considered weak. acid and base chart lists the. Is Hf Stronger Than Hcn.
From www.slideserve.com
PPT Chapter 10 Theories of Bonding and Structure PowerPoint Is Hf Stronger Than Hcn 33 rows acid with values less than one are considered weak. of these acids the strongest is hf as it has the largest k a. Simple to use laboratory reference chart for scientists,. for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. Hydrocyanic is the weakest of the. Is Hf Stronger Than Hcn.
From www.chegg.com
Solved The substance HF is seen to be a stronger acid than Is Hf Stronger Than Hcn acid and base chart lists the strength of acids and bases (strongest to weakest) in order. Hydrocyanic is the weakest of the group as it has the smallest k a. hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. of these acids the strongest is hf as it has the. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVEDBe sure to answer all parts Label the stronger acid in each Is Hf Stronger Than Hcn determine the ionization constant of \(\ce{nh4+}\), and decide which is the stronger acid, hcn or \(\ce{nh4+}\). The strong bases are listed at the bottom right of the table and. for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. Hydrocyanic is the weakest of the group as it has the. Is Hf Stronger Than Hcn.
From www.chegg.com
Solved (1) (2 pts) HF(aq) is a stronger acid than HCN(aq). Is Hf Stronger Than Hcn 33 rows acid with values less than one are considered weak. for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about a million times stronger acid than. The strong bases are listed at the bottom right of the table and. of these acids the strongest is hf as it has the largest k a. . Is Hf Stronger Than Hcn.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation ID6155898 Is Hf Stronger Than Hcn Hydrocyanic is the weakest of the group as it has the smallest k a. As the concentration of hydrofluoric acid approaches 100 percent, it's acidity increases because of homoassociation, where a base and conjugate acid form a bond: The strong bases are listed at the bottom right of the table and. 33 rows acid with values less than one. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVED Given the following information hypochlorous acid = HClO Is Hf Stronger Than Hcn Hydrocyanic is the weakest of the group as it has the smallest k a. 33 rows acid with values less than one are considered weak. of these acids the strongest is hf as it has the largest k a. the enthalpy change for hf is much smaller in magnitude than that for the other three hydrogen halides,. Is Hf Stronger Than Hcn.
From www.chegg.com
Solved 2. HF(aq) is a stronger acid than HCN(aq). For the Is Hf Stronger Than Hcn determine the ionization constant of [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex], and decide which is the stronger acid, hcn or [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex]. 33 rows acid with values less than one are considered weak. Simple to use laboratory reference chart for scientists,. As the concentration of hydrofluoric acid approaches 100 percent, it's acidity increases because of homoassociation, where a base and conjugate acid form a. Is Hf Stronger Than Hcn.
From techiescientist.com
HF Lewis Structure, Molecular Geometry, Hybridization, and Polarity Is Hf Stronger Than Hcn hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. acid and base chart lists the strength of acids and bases (strongest to weakest) in order. The strong bases are listed at the bottom right of the table and. for example, nitrous acid (\(hno_2\)), with a \(pk_a\) of 3.25, is about. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVED Given the following information acetic acid = CH3COOH Is Hf Stronger Than Hcn Simple to use laboratory reference chart for scientists,. hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. determine the ionization constant of [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex], and decide which is the stronger acid, hcn or [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex]. of these acids the strongest is hf as it has the largest k a. The strong bases. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVEDUsing Table 14.4, identify the stronger acid in each of the Is Hf Stronger Than Hcn determine the ionization constant of [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex], and decide which is the stronger acid, hcn or [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex]. acid and base chart lists the strength of acids and bases (strongest to weakest) in order. hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. of these acids the strongest is hf as. Is Hf Stronger Than Hcn.
From www.chegg.com
Solved Question 12 1 pts HF is a stronger acid than HCN. Is Hf Stronger Than Hcn 33 rows acid with values less than one are considered weak. The strong bases are listed at the bottom right of the table and. determine the ionization constant of [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex], and decide which is the stronger acid, hcn or [latex]{\text{nh}}_{4}{}^{\text{+}}[/latex]. determine the ionization constant of \(\ce{nh4+}\), and decide which is the stronger acid, hcn or \(\ce{nh4+}\). Hydrocyanic. Is Hf Stronger Than Hcn.
From www.numerade.com
SOLVED Question 18 Weak acid H3PO4, HCN, H2CO3, HCOOH, HF, CH3COOH Is Hf Stronger Than Hcn As the concentration of hydrofluoric acid approaches 100 percent, it's acidity increases because of homoassociation, where a base and conjugate acid form a bond: The strong bases are listed at the bottom right of the table and. hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. 33 rows acid with values. Is Hf Stronger Than Hcn.