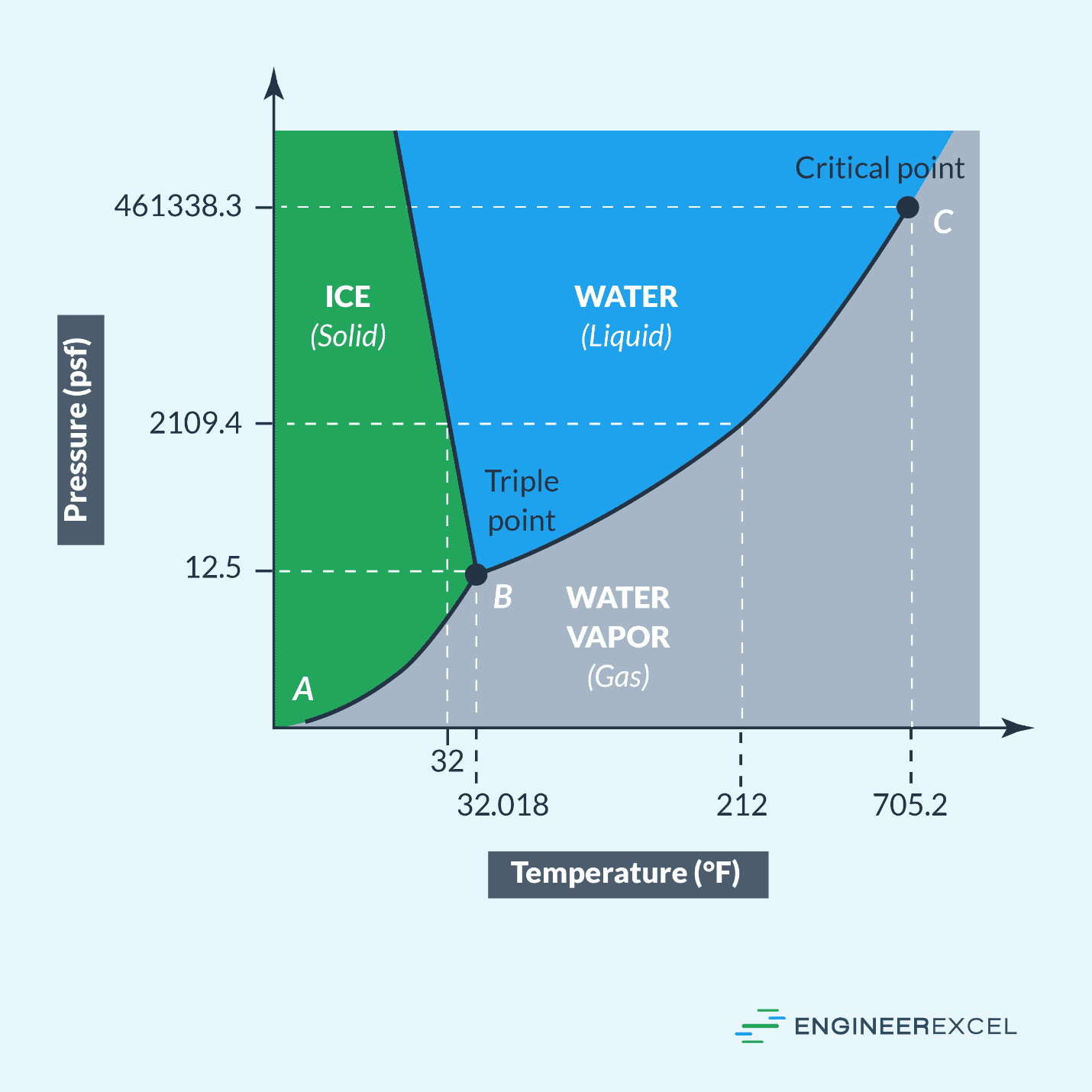
Does Temperature Increase Pressure . The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). To do that you can either add more particles into the same container, reduced the volume of the. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. This is a temperature scale where temperature and pressure are directly related to one another. In reality, most compression take place by reducing volume. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. Pressure increases when particles move faster. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general:
from engineerexcel.com
A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). In reality, most compression take place by reducing volume. To do that you can either add more particles into the same container, reduced the volume of the. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Pressure increases when particles move faster. This is a temperature scale where temperature and pressure are directly related to one another.
Pressure Temperature Graphs Explained EngineerExcel
Does Temperature Increase Pressure Pressure increases when particles move faster. Pressure increases when particles move faster. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. This is a temperature scale where temperature and pressure are directly related to one another. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. To do that you can either add more particles into the same container, reduced the volume of the. In reality, most compression take place by reducing volume.
From www.youtube.com
The Effect of Temperature and Pressure on Solubility Mr C YouTube Does Temperature Increase Pressure To do that you can either add more particles into the same container, reduced the volume of the. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. These examples of the effect of temperature on the volume of a given amount of a confined gas at constant. Does Temperature Increase Pressure.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint Does Temperature Increase Pressure If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. This is a temperature scale where temperature and pressure are directly. Does Temperature Increase Pressure.
From docslib.org
Relationship Between Density, Pressure, and Temperature DocsLib Does Temperature Increase Pressure A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). This is a temperature scale where temperature and pressure are directly related to one another. To do that you can either add more particles into the same container, reduced the volume of the. Pressure increases when particles move faster. Increasing temperature increases the average kinetic energy (ke) and. Does Temperature Increase Pressure.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Does Temperature Increase Pressure If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: In reality, most compression take place by reducing volume. This is a temperature. Does Temperature Increase Pressure.
From slidetodoc.com
The relationship between temperature and volume How Volume Does Temperature Increase Pressure These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). Pressure increases when particles move faster. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting. Does Temperature Increase Pressure.
From brainly.in
diagram of relation between pressure and temperature Brainly.in Does Temperature Increase Pressure The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). This is a temperature scale where temperature and pressure are directly related to one another. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. To do that you. Does Temperature Increase Pressure.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Temperature Increase Pressure A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). To do that you can either add more particles into the same container, reduced the volume of the. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. This is a temperature scale where temperature and. Does Temperature Increase Pressure.
From www.nagwa.com
Question Video Determining the Pressure Change of a Gas When Its Does Temperature Increase Pressure This is a temperature scale where temperature and pressure are directly related to one another. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. To do that you. Does Temperature Increase Pressure.
From www.slideserve.com
PPT Fluid Mechanics PowerPoint Presentation, free download ID6711078 Does Temperature Increase Pressure Pressure increases when particles move faster. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). To do that you can either add more particles into the same container, reduced the volume of the. In reality,. Does Temperature Increase Pressure.
From socratic.org
How do mountains affect temperature on both the windward side and the Does Temperature Increase Pressure To do that you can either add more particles into the same container, reduced the volume of the. These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). In reality, most compression take. Does Temperature Increase Pressure.
From mungfali.com
Maxwell Boltzmann Distribution Temperature Does Temperature Increase Pressure A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). In reality, most compression take place by reducing volume. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. This is a temperature scale where temperature and pressure are directly related to one another. The relationships. Does Temperature Increase Pressure.
From chem.libretexts.org
13.4 Pressure and Temperature Effects on Solubility Chemistry LibreTexts Does Temperature Increase Pressure This is a temperature scale where temperature and pressure are directly related to one another. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Pressure increases when particles move faster. These examples of the effect of temperature on the volume of a given amount of a confined. Does Temperature Increase Pressure.
From pediaa.com
Relationship Between Pressure and Temperature Does Temperature Increase Pressure This is a temperature scale where temperature and pressure are directly related to one another. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). If you had a way to increase pressure with no volume change, then yes,. Does Temperature Increase Pressure.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Does Temperature Increase Pressure A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: In reality, most compression take place by reducing volume. The relationships among the volume of a gas and its pressure, temperature, and amount. Does Temperature Increase Pressure.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Does Temperature Increase Pressure In reality, most compression take place by reducing volume. These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). If you had a way to increase pressure with no volume change, then yes,. Does Temperature Increase Pressure.
From saylordotorg.github.io
Relationships among Pressure, Temperature, Volume, and Amount Does Temperature Increase Pressure In reality, most compression take place by reducing volume. Pressure increases when particles move faster. This is a temperature scale where temperature and pressure are directly related to one another. To do that you can either add more particles into the same container, reduced the volume of the. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \).. Does Temperature Increase Pressure.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Does Temperature Increase Pressure Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. Pressure increases when particles move faster. To do that you can either add more particles into the same container, reduced the volume of the. If you had a way to increase pressure with. Does Temperature Increase Pressure.
From www.quora.com
If temperature increases, pressure increases. Does temperature increase Does Temperature Increase Pressure This is a temperature scale where temperature and pressure are directly related to one another. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. In reality, most compression take place by reducing volume. These examples of the effect of temperature on the volume of a given amount. Does Temperature Increase Pressure.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Temperature Increase Pressure If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. To do that you can either add more particles into the same container, reduced the volume of the. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). This. Does Temperature Increase Pressure.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Does Temperature Increase Pressure In reality, most compression take place by reducing volume. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. These examples. Does Temperature Increase Pressure.
From slideplayer.com
Aim How does temperature affect the vapor pressure of liquids Does Temperature Increase Pressure This is a temperature scale where temperature and pressure are directly related to one another. To do that you can either add more particles into the same container, reduced the volume of the. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). In reality, most compression take place by reducing volume. Increasing temperature increases the average kinetic. Does Temperature Increase Pressure.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica Does Temperature Increase Pressure To do that you can either add more particles into the same container, reduced the volume of the. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). This is a temperature scale where temperature and pressure are directly related to one another. Pressure increases when particles move faster. In reality, most compression take place by reducing volume.. Does Temperature Increase Pressure.
From www.youtube.com
Effects of Temperature and Pressure on Matter iKen iKen Edu iKen Does Temperature Increase Pressure A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). If you. Does Temperature Increase Pressure.
From courses.lumenlearning.com
Phase Changes Physics Does Temperature Increase Pressure These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). This is a temperature scale where temperature and pressure are directly related to one another. Pressure increases when particles move faster. To do. Does Temperature Increase Pressure.
From www.youtube.com
How to Answer Equilibrium Graph Exam Questions // HSC Chemistry YouTube Does Temperature Increase Pressure Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). This is a temperature scale where temperature and pressure are directly related to one. Does Temperature Increase Pressure.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1757364 Does Temperature Increase Pressure Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. In reality, most compression take place by reducing volume. To do. Does Temperature Increase Pressure.
From exoudqbxz.blob.core.windows.net
Does Temperature Increase When Pressure Increases at Hollis Winter blog Does Temperature Increase Pressure In reality, most compression take place by reducing volume. These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: Pressure increases when particles move faster. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent. Does Temperature Increase Pressure.
From gcsephysicsninja.com
11. Heating gas at a constant pressure Does Temperature Increase Pressure To do that you can either add more particles into the same container, reduced the volume of the. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. These examples of the effect of temperature on the volume of a given amount of. Does Temperature Increase Pressure.
From www.slideserve.com
PPT Characteristics of the Atmosphere PowerPoint Presentation, free Does Temperature Increase Pressure In reality, most compression take place by reducing volume. This is a temperature scale where temperature and pressure are directly related to one another. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. The relationships among the volume of a gas and its pressure, temperature, and amount. Does Temperature Increase Pressure.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Does Temperature Increase Pressure Pressure increases when particles move faster. In reality, most compression take place by reducing volume. These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent. Does Temperature Increase Pressure.
From www.slideserve.com
PPT Chapter 14 PowerPoint Presentation, free download ID3552130 Does Temperature Increase Pressure This is a temperature scale where temperature and pressure are directly related to one another. These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: To do that you can either add more particles into the same container, reduced the volume of the. The relationships. Does Temperature Increase Pressure.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Does Temperature Increase Pressure These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. In reality, most compression take place by reducing volume. To do that you. Does Temperature Increase Pressure.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Does Temperature Increase Pressure Pressure increases when particles move faster. These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). In reality, most compression take place by reducing volume. To. Does Temperature Increase Pressure.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Does Temperature Increase Pressure A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). To do that you can either add more particles into the same container, reduced the volume of the. These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true in general: In reality, most compression take. Does Temperature Increase Pressure.
From www.esa.int
ESA Atmospheric temperature changes with altitude Does Temperature Increase Pressure A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. These examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure. Does Temperature Increase Pressure.