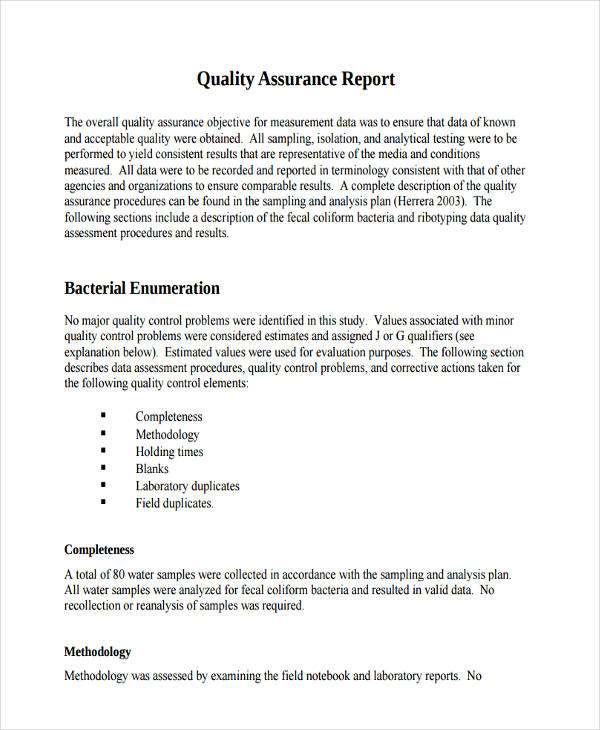
Recording And Reporting Of Quality . This document is intended to provide information about: Write an sop for recording, reporting and archiving of results. This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. The complexity of the quality measure development process, which is. Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. This sop must cover the following procedures:
from www.examples.com
This sop must cover the following procedures: This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. The complexity of the quality measure development process, which is. Write an sop for recording, reporting and archiving of results. This document is intended to provide information about:
Quality Report 18+ Examples, Format, How To Create, Pdf
Recording And Reporting Of Quality Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. This sop must cover the following procedures: This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. This document is intended to provide information about: Write an sop for recording, reporting and archiving of results. The complexity of the quality measure development process, which is. Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to.
From www.slideteam.net
Bpo Call Monitoring Recording Scripting Quality Parameters PowerPoint Slide Clipart Example Recording And Reporting Of Quality Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. This document is intended. Recording And Reporting Of Quality.
From www.imageapi.com
Why is Records Management Important? [Top 8 Benefits] Recording And Reporting Of Quality Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. The complexity of the quality measure development process, which is. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. Write an sop for recording, reporting and archiving of results. This chapter focuses on. Recording And Reporting Of Quality.
From www.youtube.com
Recordkeeping and Reporting YouTube Recording And Reporting Of Quality This sop must cover the following procedures: Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. Clinical record (cr). Recording And Reporting Of Quality.
From criticalthinking.cloud
how to write a quality management report Recording And Reporting Of Quality Write an sop for recording, reporting and archiving of results. Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. This sop must cover the following procedures: This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. Records are the collected information produced by the. Recording And Reporting Of Quality.
From www.slideserve.com
PPT Chapter 9 Recording and Reporting PowerPoint Presentation, free download ID9562389 Recording And Reporting Of Quality Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. Write an sop for. Recording And Reporting Of Quality.
From www.examples.com
Quality Report 18+ Examples, Format, How To Create, Pdf Recording And Reporting Of Quality Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. The complexity of the quality measure development process, which is. Records are the collected information produced by the laboratory in the process of performing and. Recording And Reporting Of Quality.
From doctemplates.us
17+ Quality Control Report Template DocTemplates Recording And Reporting Of Quality This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. Write an sop for recording, reporting and archiving of results. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing,. Recording And Reporting Of Quality.
From www.slideserve.com
PPT Recording and Reporting PowerPoint Presentation, free download ID5700061 Recording And Reporting Of Quality This document is intended to provide information about: The complexity of the quality measure development process, which is. Write an sop for recording, reporting and archiving of results. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. This sop must cover the following procedures: Records are the collected information produced by the. Recording And Reporting Of Quality.
From www.slideshare.net
Recording & reporting Recording And Reporting Of Quality This sop must cover the following procedures: Write an sop for recording, reporting and archiving of results. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. The complexity of the quality measure. Recording And Reporting Of Quality.
From www.health.gov.au
National Aged Care Mandatory Quality Indicator Program (QI Program) Australian Government Recording And Reporting Of Quality This document is intended to provide information about: Write an sop for recording, reporting and archiving of results. Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. The complexity of. Recording And Reporting Of Quality.
From www.en-standard.eu
ISO 18400107 European Standards Recording And Reporting Of Quality This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. Write an sop for recording, reporting and archiving of results. Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information. Recording And Reporting Of Quality.
From lesboucans.com
Quality Control Reports Template Collection Recording And Reporting Of Quality This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. Write an sop for recording, reporting and archiving of results. This sop must cover the following procedures: The complexity of the quality measure development process, which is. Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its. Recording And Reporting Of Quality.
From slidesdocs.com
Quality Inspection Report With Quality Inspection Statistics Excel Template And Google Sheets Recording And Reporting Of Quality This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. Write an sop for recording, reporting and archiving of results. This sop must cover the following procedures: Primary care practices are being required. Recording And Reporting Of Quality.
From www.slideteam.net
Four Stages Of Record To Report Process PPT Presentation Recording And Reporting Of Quality Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. This sop must cover the following procedures: Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. Write an sop for recording, reporting and archiving of results. Clinical record (cr) is the primary. Recording And Reporting Of Quality.
From www.inpaspages.com
Quality inspection history record Recording And Reporting Of Quality Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. Records are the collected information produced by the laboratory in. Recording And Reporting Of Quality.
From www.slideteam.net
Top 5 Quality Inspection Report Templates with Samples and Examples Recording And Reporting Of Quality The complexity of the quality measure development process, which is. This document is intended to provide information about: Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. This sop must cover the following procedures:. Recording And Reporting Of Quality.
From www.examples.com
Quality Report 18+ Examples, Format, How To Create, Pdf Recording And Reporting Of Quality Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. The complexity of the quality measure development process, which is. Write an sop for recording, reporting and archiving of results. This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. Primary care practices are being. Recording And Reporting Of Quality.
From studylib.net
Assessing recording and reporting Recording and reporting Recording And Reporting Of Quality Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. The complexity of the quality measure development process, which. Recording And Reporting Of Quality.
From www.slideshare.net
Recording & reporting Recording And Reporting Of Quality This document is intended to provide information about: The complexity of the quality measure development process, which is. This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. This sop must cover the following procedures: Write an sop for recording, reporting and archiving of results. Records are the collected information produced by the laboratory in. Recording And Reporting Of Quality.
From www.osmosis.org
Documentation and reporting Biblioteca de Osmosis Recording And Reporting Of Quality This document is intended to provide information about: Write an sop for recording, reporting and archiving of results. Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. Clinical record (cr) is the primary. Recording And Reporting Of Quality.
From www.slideserve.com
PPT Introduction to the DoD Exposure Assessment Model and DOEHRSIH PowerPoint Presentation Recording And Reporting Of Quality This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. Write an sop for recording, reporting and archiving of results. The complexity of the quality measure development process, which is. This document is intended to provide information about: Records are the collected information produced by the laboratory in the process of performing and reporting a. Recording And Reporting Of Quality.
From www.researchgate.net
(PDF) Quality of patient record keeping An indicator of the quality of care? Recording And Reporting Of Quality This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. The complexity of the quality measure development process, which is. Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and. Recording And Reporting Of Quality.
From www.examples.com
Quality Report 18+ Examples, Format, How To Create, Pdf Recording And Reporting Of Quality This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. Write an sop for recording, reporting and archiving of results. This document is intended to provide information about: Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. Primary care practices are being required to capture. Recording And Reporting Of Quality.
From www.youtube.com
RECORD AND REPORT DOCUMENTATION RECORDING AND REPORTING CHARACTERISTICS OF GOOD RECORD Recording And Reporting Of Quality This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. This document is intended to provide information about: Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. The complexity. Recording And Reporting Of Quality.
From www.slideshare.net
Recording & reporting Recording And Reporting Of Quality The complexity of the quality measure development process, which is. This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,.. Recording And Reporting Of Quality.
From sitemate.com
QC Report template (Better format than excel) Free to use Recording And Reporting Of Quality Write an sop for recording, reporting and archiving of results. The complexity of the quality measure development process, which is. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. Clinical record (cr) is. Recording And Reporting Of Quality.
From www.scribd.com
Guide to Using the WCED Recording and Reporting Programme Microsoft Excel Areas Of Computer Recording And Reporting Of Quality Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. This document is intended to. Recording And Reporting Of Quality.
From www.slideshare.net
Recording & reporting Recording And Reporting Of Quality This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. The complexity of the quality measure development process, which is. Records are the collected information produced by the laboratory in the process of performing and reporting a laboratory test. This document addresses the good clinical practice, an international ethical and scientific quality standard for designing,. Recording And Reporting Of Quality.
From china.docshipper.com
[Free Report] Product Quality Inspection (QC) Example Recording And Reporting Of Quality This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,. The complexity of the quality measure development process, which is. Write an sop for recording, reporting and archiving of results. Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. This document is intended to. Recording And Reporting Of Quality.
From insight-quality.com
Product Quality Inspection Report Sample Insight Quality Services Recording And Reporting Of Quality This document is intended to provide information about: Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. This chapter focuses on the procedures for obtaining registry data and associated quality assurance. Recording And Reporting Of Quality.
From studylib.net
Standard Operating Procedure for the Recording and Reporting of Recording And Reporting Of Quality This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. Write an sop for recording, reporting and archiving of results. This sop must cover the following procedures: Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. This document is intended to provide information about: Records are. Recording And Reporting Of Quality.
From www.allbusinesstemplates.com
Product Quality Inspection Report Template Templates at Recording And Reporting Of Quality The complexity of the quality measure development process, which is. This sop must cover the following procedures: Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. This document addresses the good. Recording And Reporting Of Quality.
From www.template.net
FREE Quality Report Templates Download in Word, Google Docs, Excel, PDF, Google Sheets Recording And Reporting Of Quality This document is intended to provide information about: This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. Clinical record (cr) is the primary tool used by healthcare workers (hcws) to record clinical information and its completeness can. The complexity of the quality measure development process, which is. This document addresses the good clinical practice,. Recording And Reporting Of Quality.
From www.slideshare.net
Recording & reporting Recording And Reporting Of Quality This chapter focuses on the procedures for obtaining registry data and associated quality assurance principles. This document is intended to provide information about: Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. The complexity of the quality measure development process, which is. This sop must cover the following procedures: This document. Recording And Reporting Of Quality.
From www.slideteam.net
Top 5 Quality Report Templates with Samples and Examples Recording And Reporting Of Quality This document is intended to provide information about: The complexity of the quality measure development process, which is. Primary care practices are being required to capture and report results from electronic clinical quality measures (ecqms) to. This sop must cover the following procedures: This document addresses the good clinical practice, an international ethical and scientific quality standard for designing, conducting,.. Recording And Reporting Of Quality.