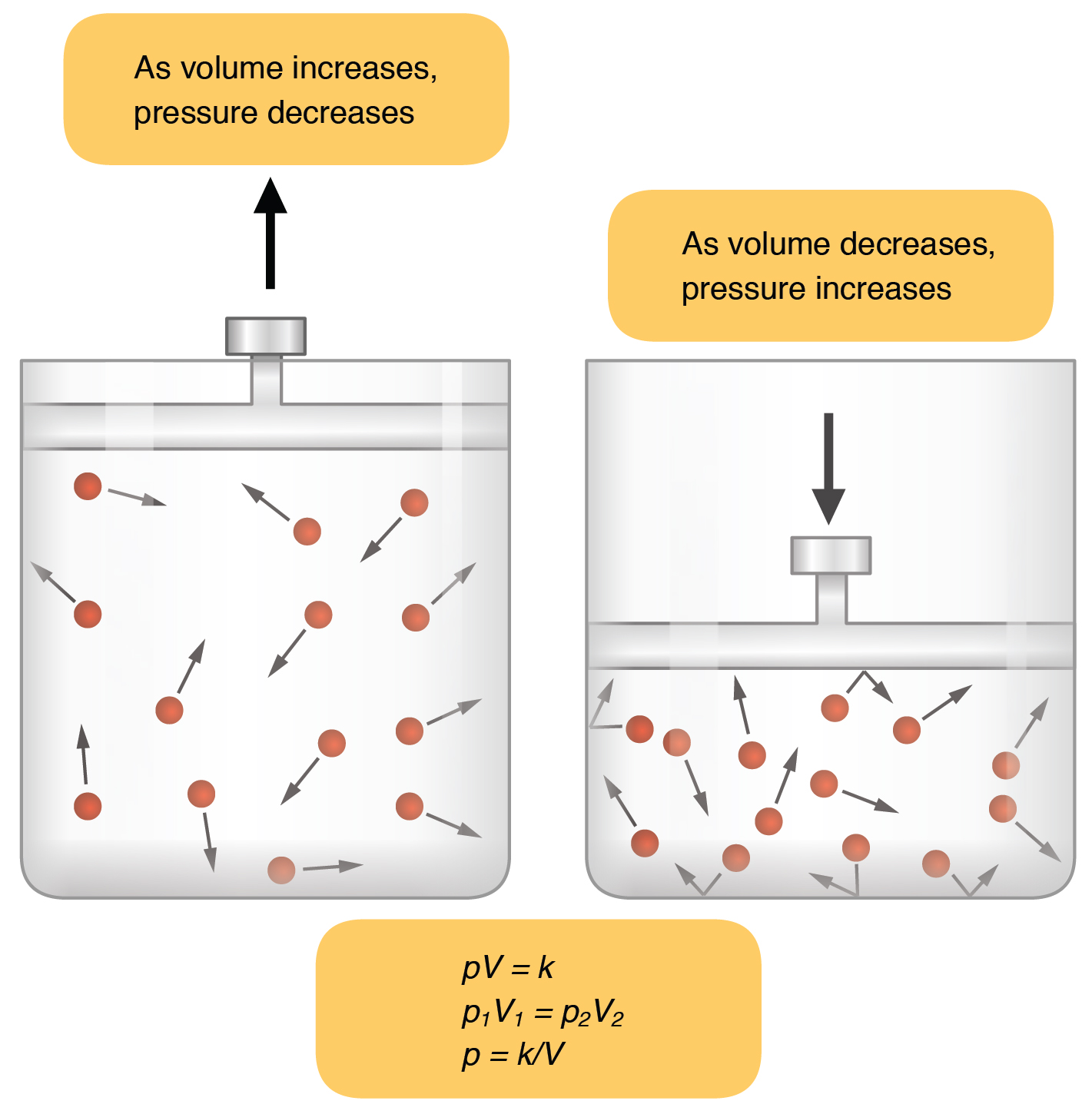
Gas Law Temp And Pressure . the pressure of a gas is inversely proportional to its volume when temperature is constant. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. boyle’s law describes the inverse proportional relationship between pressure and volume at a constant.
from socratic.org
boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the pressure of a gas is inversely proportional to its volume when temperature is constant. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to.
A sample of a gas has a volume of 2.0 liters at a pressure of 1.0
Gas Law Temp And Pressure the pressure of a gas is inversely proportional to its volume when temperature is constant. the pressure of a gas is inversely proportional to its volume when temperature is constant. boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Gas Law Temp And Pressure the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in. Gas Law Temp And Pressure.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal Gas Law Temp And Pressure use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. the pressure of a gas is inversely proportional to its volume when temperature is constant. boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. the combined gas. Gas Law Temp And Pressure.
From printablelibhavre.z21.web.core.windows.net
Simple Explanation Of Boyle's Law Gas Law Temp And Pressure the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. the pressure of a gas is inversely proportional to its volume when temperature is constant. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. use the ideal gas law. Gas Law Temp And Pressure.
From learningmagicharlow.z13.web.core.windows.net
All Gas Law Formulas Gas Law Temp And Pressure the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. the pressure of a gas is inversely proportional to its volume when temperature is constant. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. boyle’s law describes the inverse. Gas Law Temp And Pressure.
From www.chegg.com
The ideal gas law relates the pressure, density, and Gas Law Temp And Pressure the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. the pressure of a gas is inversely proportional to its volume when temperature is constant. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. Gas Law Temp And Pressure.
From www.youtube.com
11.6 The Combined Gas Law Pressure, Volume, & Temperature YouTube Gas Law Temp And Pressure the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in. Gas Law Temp And Pressure.
From sciencenotes.org
Ideal Gas Law Formula and Examples Gas Law Temp And Pressure the pressure of a gas is inversely proportional to its volume when temperature is constant. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. Gas Law Temp And Pressure.
From mmerevise.co.uk
The Ideal Gas Equation MME Gas Law Temp And Pressure the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. use the ideal gas law to. Gas Law Temp And Pressure.
From www.grc.nasa.gov
Equation of State Gas Law Temp And Pressure the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. the pressure of a gas is inversely proportional to its volume when temperature is constant. use the ideal gas law to calculate. Gas Law Temp And Pressure.
From www.pinterest.co.kr
Ideal Gas Law relationship between pressure, temperature, volume Gas Law Temp And Pressure the pressure of a gas is inversely proportional to its volume when temperature is constant. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. boyle’s. Gas Law Temp And Pressure.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal Gas Law Temp And Pressure the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in. Gas Law Temp And Pressure.
From app.pandai.org
Gas Laws Gas Law Temp And Pressure boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. the pressure of a gas is inversely proportional to its volume when temperature is constant. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. use the ideal gas law to calculate. Gas Law Temp And Pressure.
From socratic.org
A sample of a gas has a volume of 2.0 liters at a pressure of 1.0 Gas Law Temp And Pressure the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. the combined. Gas Law Temp And Pressure.
From www.met.reading.ac.uk
PPLATO FLAP PHYS 7.2 Temperature, pressure and the ideal gas laws Gas Law Temp And Pressure the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. the pressure of a gas is inversely proportional to its volume when temperature is constant. boyle’s law describes the inverse. Gas Law Temp And Pressure.
From fyosafgjg.blob.core.windows.net
Gas Law Temperature And Volume at Michael Freeman blog Gas Law Temp And Pressure the pressure of a gas is inversely proportional to its volume when temperature is constant. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. the combined gas. Gas Law Temp And Pressure.
From owlcation.com
The Theories and Behavior of Gas Owlcation Gas Law Temp And Pressure the pressure of a gas is inversely proportional to its volume when temperature is constant. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. the combined gas. Gas Law Temp And Pressure.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Gas Law Temp And Pressure the pressure of a gas is inversely proportional to its volume when temperature is constant. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. Gas Law Temp And Pressure.
From chemistry101efhs.weebly.com
Gas Laws Chemistry 101 Gas Law Temp And Pressure the pressure of a gas is inversely proportional to its volume when temperature is constant. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. Gas Law Temp And Pressure.
From chem.libretexts.org
6.3 The Gas Laws Chemistry LibreTexts Gas Law Temp And Pressure boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given. Gas Law Temp And Pressure.
From www.alamy.com
Charles's law. relationship between volume and temperature. Increasing Gas Law Temp And Pressure use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. the pressure of a gas is inversely proportional to its volume when temperature is constant. the three fundamental. Gas Law Temp And Pressure.
From cartoondealer.com
Boyle's Law, Relationship Between Pressure And Volume Of Gas At Gas Law Temp And Pressure use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the pressure. Gas Law Temp And Pressure.
From owlcation.com
The Theories and Behavior of Gas Owlcation Gas Law Temp And Pressure the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the pressure of a gas is inversely proportional to its volume when temperature is constant. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. boyle’s. Gas Law Temp And Pressure.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Gas Law Temp And Pressure boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional. Gas Law Temp And Pressure.
From einmalalle.blogspot.com
Ideal Gas Law R Values / Why are Gas Laws Important in Chemistry Gas Law Temp And Pressure the pressure of a gas is inversely proportional to its volume when temperature is constant. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. the combined gas law shows that the pressure of a gas. Gas Law Temp And Pressure.
From www.askiitians.com
Gas Laws And Properties Of Gases Study Material for IIT JEE askIITians Gas Law Temp And Pressure the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. the pressure of a gas is inversely proportional to its volume when temperature is constant. the. Gas Law Temp And Pressure.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Gas Law Temp And Pressure the pressure of a gas is inversely proportional to its volume when temperature is constant. boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. the combined gas. Gas Law Temp And Pressure.
From www.youtube.com
11.8 The Ideal Gas Law Pressure, Volume, Temperature, & Moles YouTube Gas Law Temp And Pressure the pressure of a gas is inversely proportional to its volume when temperature is constant. boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. use the ideal gas law to calculate. Gas Law Temp And Pressure.
From fineartamerica.com
Gay Lussac's Pressuretemperature Gas Law Photograph by Science Photo Gas Law Temp And Pressure the pressure of a gas is inversely proportional to its volume when temperature is constant. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a. Gas Law Temp And Pressure.
From drcalef.com
The Combined Gas Law Pressure, Volume, and Temperature Gas Law Temp And Pressure the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. the pressure of a gas is inversely proportional to its volume when temperature is constant. boyle’s. Gas Law Temp And Pressure.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica Gas Law Temp And Pressure use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. the pressure of a gas is inversely proportional to its volume when temperature. Gas Law Temp And Pressure.
From www.youtube.com
Combined Gas Law Pressure, Volume and Temperature Straight Science Gas Law Temp And Pressure boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given. Gas Law Temp And Pressure.
From www.chegg.com
Solved The graph below shows the ideal gas law relationship Gas Law Temp And Pressure boyle’s law describes the inverse proportional relationship between pressure and volume at a constant. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given. Gas Law Temp And Pressure.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Gas Law Temp And Pressure the pressure of a gas is inversely proportional to its volume when temperature is constant. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. boyle’s law describes the inverse. Gas Law Temp And Pressure.
From www.youtube.com
LM Unit 9 Intro Ideal Gas Law YouTube Gas Law Temp And Pressure the pressure of a gas is inversely proportional to its volume when temperature is constant. use the ideal gas law to calculate pressure change, temperature change, volume change, or the number of molecules or moles in a given volume. the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly. Gas Law Temp And Pressure.
From study.com
GayLussac's Law Gas Pressure and Temperature Relationship Video Gas Law Temp And Pressure the combined gas law shows that the pressure of a gas is inversely proportional to volume and directly proportional to. the pressure of a gas is inversely proportional to its volume when temperature is constant. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. boyle’s law describes the inverse. Gas Law Temp And Pressure.