Electron Configuration For Aluminum Atomic Number 13 Is . The atomic number gives us the number of protons in an atom of aluminum. Atomic mass, electron configurations, charges, and more. Get a free hd image of the periodic. Since the 1s orbital can hold only two electrons the next two enter the 2s orbital. Access detailed info on all elements: To find the electron configuration of an atom, you first need to know the number of electrons that it has. View rotating bohr models for all 118 elements. The element with atomic number 13 is aluminum. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. A neutral atom of aluminum has 13 protons and 13 electrons. Aluminum’s atomic number is 13, which means its atom has thirteen electrons around its nucleus. To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. The atomic number of aluminum is 13. The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1.
from ar.inspiredpencil.com
Aluminum’s atomic number is 13, which means its atom has thirteen electrons around its nucleus. The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. Atomic mass, electron configurations, charges, and more. The element with atomic number 13 is aluminum. View rotating bohr models for all 118 elements. A neutral atom of aluminum has 13 protons and 13 electrons. To find the electron configuration of an atom, you first need to know the number of electrons that it has. Since the 1s orbital can hold only two electrons the next two enter the 2s orbital. Get a free hd image of the periodic. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13.
Electron Configuration Of Aluminum
Electron Configuration For Aluminum Atomic Number 13 Is Access detailed info on all elements: A neutral atom of aluminum has 13 protons and 13 electrons. To find the electron configuration of an atom, you first need to know the number of electrons that it has. Atomic mass, electron configurations, charges, and more. Since the 1s orbital can hold only two electrons the next two enter the 2s orbital. The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. The element with atomic number 13 is aluminum. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. Get a free hd image of the periodic. Access detailed info on all elements: Aluminum’s atomic number is 13, which means its atom has thirteen electrons around its nucleus. The atomic number of aluminum is 13. View rotating bohr models for all 118 elements. To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. The atomic number gives us the number of protons in an atom of aluminum.
From ar.inspiredpencil.com
Aluminium Electron Configuration Electron Configuration For Aluminum Atomic Number 13 Is To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. Since the 1s orbital can hold only two electrons the next two enter the 2s orbital. The element with atomic number 13 is aluminum. Access detailed info on all elements: Get a free hd image of the periodic. View rotating bohr models for all 118. Electron Configuration For Aluminum Atomic Number 13 Is.
From mavink.com
Aluminum Shell Diagram Electron Configuration For Aluminum Atomic Number 13 Is Get a free hd image of the periodic. Atomic mass, electron configurations, charges, and more. Access detailed info on all elements: The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. View rotating bohr models for all 118 elements. Aluminum’s atomic number is 13, which means its atom has thirteen electrons. Electron Configuration For Aluminum Atomic Number 13 Is.
From www.vrogue.co
Aluminum Facts Atomic Number 13 Or Al vrogue.co Electron Configuration For Aluminum Atomic Number 13 Is Access detailed info on all elements: View rotating bohr models for all 118 elements. To find the electron configuration of an atom, you first need to know the number of electrons that it has. The element with atomic number 13 is aluminum. A neutral atom of aluminum has 13 protons and 13 electrons. The atomic number gives us the number. Electron Configuration For Aluminum Atomic Number 13 Is.
From www.alamy.com
3d render of atom structure of aluminum isolated over white background Electron Configuration For Aluminum Atomic Number 13 Is Atomic mass, electron configurations, charges, and more. Get a free hd image of the periodic. To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. Access detailed info on all elements:. Electron Configuration For Aluminum Atomic Number 13 Is.
From material-properties.org
Aluminium Protons Neutrons Electrons Electron Configuration Electron Configuration For Aluminum Atomic Number 13 Is Get a free hd image of the periodic. Since the 1s orbital can hold only two electrons the next two enter the 2s orbital. To find the electron configuration of an atom, you first need to know the number of electrons that it has. The atomic number of aluminum is 13. To write the electron configuration for aluminum, the first. Electron Configuration For Aluminum Atomic Number 13 Is.
From www.alamy.com
Al Aluminium Chemical Element Periodic Table. Single vector Electron Configuration For Aluminum Atomic Number 13 Is A neutral atom of aluminum has 13 protons and 13 electrons. View rotating bohr models for all 118 elements. Since the 1s orbital can hold only two electrons the next two enter the 2s orbital. Access detailed info on all elements: In order to write the aluminium electron configuration we first need to know the number of electrons for the. Electron Configuration For Aluminum Atomic Number 13 Is.
From www.sciencephoto.com
Aluminium, atomic structure Stock Image C013/1526 Science Photo Library Electron Configuration For Aluminum Atomic Number 13 Is The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. Atomic mass, electron configurations, charges, and more. The atomic number of aluminum is 13. Access detailed info on all elements: In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom. Electron Configuration For Aluminum Atomic Number 13 Is.
From brainly.ph
Al13 orbital diagram and Electron Configuration Brainly.ph Electron Configuration For Aluminum Atomic Number 13 Is The atomic number of aluminum is 13. To find the electron configuration of an atom, you first need to know the number of electrons that it has. Atomic mass, electron configurations, charges, and more. The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. Aluminum’s atomic number is 13, which means. Electron Configuration For Aluminum Atomic Number 13 Is.
From topblogtenz.com
Aluminum Orbital diagram, Electron configuration, and Valence electrons Electron Configuration For Aluminum Atomic Number 13 Is In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. The element with atomic number 13 is aluminum. Access detailed info on all elements: Aluminum’s atomic number is 13, which means its atom has thirteen electrons around its nucleus. The atomic number gives us the number. Electron Configuration For Aluminum Atomic Number 13 Is.
From ar.inspiredpencil.com
Aluminium Electron Configuration Electron Configuration For Aluminum Atomic Number 13 Is The atomic number of aluminum is 13. The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. Access detailed info on all elements: Since the 1s orbital can hold only two electrons the next two enter. Electron Configuration For Aluminum Atomic Number 13 Is.
From ar.inspiredpencil.com
Electron Configuration Of Aluminum Electron Configuration For Aluminum Atomic Number 13 Is The atomic number of aluminum is 13. The atomic number gives us the number of protons in an atom of aluminum. The element with atomic number 13 is aluminum. Access detailed info on all elements: In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. To. Electron Configuration For Aluminum Atomic Number 13 Is.
From www.britannica.com
aluminum Uses, Properties, & Compounds Britannica Electron Configuration For Aluminum Atomic Number 13 Is The atomic number gives us the number of protons in an atom of aluminum. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. A neutral atom of aluminum has 13 protons and 13 electrons. Access detailed info on all elements: The element with atomic number. Electron Configuration For Aluminum Atomic Number 13 Is.
From mavink.com
Aluminum Orbital Diagram Electron Configuration For Aluminum Atomic Number 13 Is To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. Atomic mass, electron configurations, charges, and more. Aluminum’s atomic number is 13, which means its atom has thirteen electrons around its nucleus. To find the electron configuration of an atom, you first need to know the number of electrons that it has. A neutral atom. Electron Configuration For Aluminum Atomic Number 13 Is.
From periodictable.me
How Can We Find Electron Configuration For Aluminium (Al) Electron Configuration For Aluminum Atomic Number 13 Is The atomic number of aluminum is 13. Atomic mass, electron configurations, charges, and more. The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. In order to write the aluminium electron configuration we first need to. Electron Configuration For Aluminum Atomic Number 13 Is.
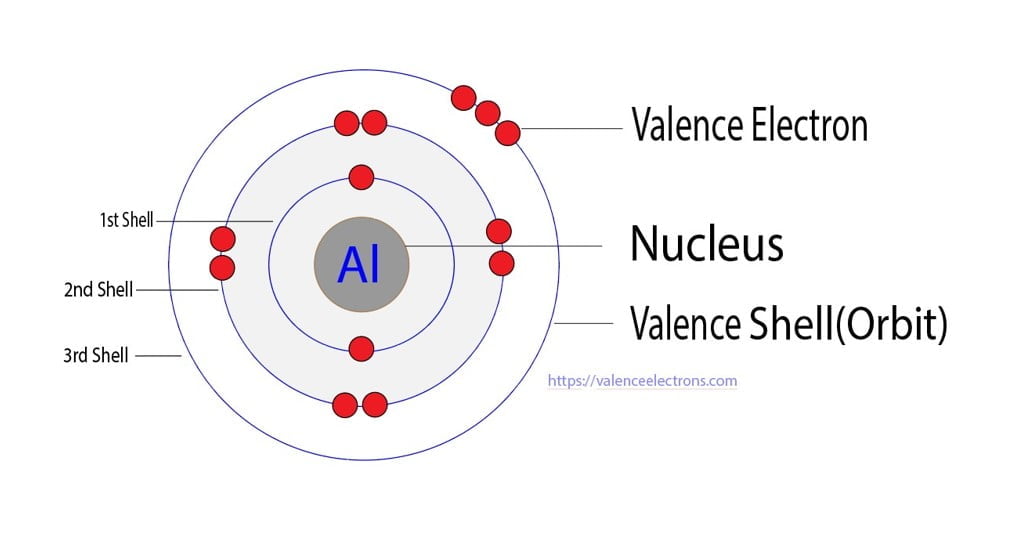
From valenceelectrons.com
How to Write the Electron Configuration for Aluminium (Al)? Electron Configuration For Aluminum Atomic Number 13 Is In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. Access detailed info on all elements: The atomic number of aluminum is 13. To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. The atomic number gives us the number of. Electron Configuration For Aluminum Atomic Number 13 Is.
From sciencenotes.org
Aluminum Atom Science Notes and Projects Electron Configuration For Aluminum Atomic Number 13 Is The element with atomic number 13 is aluminum. View rotating bohr models for all 118 elements. To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. The atomic number of aluminum is 13. Since the 1s. Electron Configuration For Aluminum Atomic Number 13 Is.
From sciencenotes.org
List of Electron Configurations of Elements Electron Configuration For Aluminum Atomic Number 13 Is A neutral atom of aluminum has 13 protons and 13 electrons. The atomic number of aluminum is 13. Get a free hd image of the periodic. To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. Atomic mass, electron configurations, charges, and more. The element with atomic number 13 is aluminum. Access detailed info on. Electron Configuration For Aluminum Atomic Number 13 Is.
From www.youtube.com
Atomic Structure (Bohr Model) for Aluminum (Al) YouTube Electron Configuration For Aluminum Atomic Number 13 Is Aluminum’s atomic number is 13, which means its atom has thirteen electrons around its nucleus. The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. Get a free hd image of the periodic. View rotating bohr. Electron Configuration For Aluminum Atomic Number 13 Is.
From www.vrogue.co
Aluminum Facts Atomic Number 13 Or Al vrogue.co Electron Configuration For Aluminum Atomic Number 13 Is The atomic number gives us the number of protons in an atom of aluminum. A neutral atom of aluminum has 13 protons and 13 electrons. View rotating bohr models for all 118 elements. The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. Access detailed info on all elements: In order. Electron Configuration For Aluminum Atomic Number 13 Is.
From sites.google.com
Aluminum Table of Elements by Shrenil Sharma Electron Configuration For Aluminum Atomic Number 13 Is Atomic mass, electron configurations, charges, and more. Get a free hd image of the periodic. A neutral atom of aluminum has 13 protons and 13 electrons. Access detailed info on all elements: The atomic number of aluminum is 13. The element with atomic number 13 is aluminum. View rotating bohr models for all 118 elements. In order to write the. Electron Configuration For Aluminum Atomic Number 13 Is.
From brainly.com
The electron configuration of aluminum, atomic number 13, is (Ne) 352 Electron Configuration For Aluminum Atomic Number 13 Is Get a free hd image of the periodic. To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. To find the electron configuration of an atom, you first need to know the number of electrons that it has. View rotating bohr models for all 118 elements. In order to write the aluminium electron configuration we. Electron Configuration For Aluminum Atomic Number 13 Is.
From www.pinterest.com
FileElectron shell 013 Aluminum.svg Wikimedia Commons Atom diagram Electron Configuration For Aluminum Atomic Number 13 Is In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. The element with atomic number 13 is aluminum. To write the electron configuration for aluminum, the first. Electron Configuration For Aluminum Atomic Number 13 Is.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Electron Configuration For Aluminum Atomic Number 13 Is The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. To find the electron configuration of an atom, you first need to know the number of electrons that it has. Aluminum’s atomic number is 13, which means its atom has thirteen electrons around its nucleus. Since the 1s orbital can hold. Electron Configuration For Aluminum Atomic Number 13 Is.
From ar.inspiredpencil.com
Electron Configuration Of Aluminum Electron Configuration For Aluminum Atomic Number 13 Is Get a free hd image of the periodic. To find the electron configuration of an atom, you first need to know the number of electrons that it has. A neutral atom of aluminum has 13 protons and 13 electrons. To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. The atomic number of aluminum is. Electron Configuration For Aluminum Atomic Number 13 Is.
From www.narodnatribuna.info
Aluminum Atomic Structure Electron Configuration For Aluminum Atomic Number 13 Is The element with atomic number 13 is aluminum. To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. Get a free hd image of the periodic. A neutral atom of aluminum. Electron Configuration For Aluminum Atomic Number 13 Is.
From www.newtondesk.com
Aluminium Al (Element 13) of Periodic Table Elements FlashCards Electron Configuration For Aluminum Atomic Number 13 Is The atomic number of aluminum is 13. A neutral atom of aluminum has 13 protons and 13 electrons. To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. Get a free hd image of the periodic. The element with atomic number 13 is aluminum. The atomic number gives us the number of protons in an. Electron Configuration For Aluminum Atomic Number 13 Is.
From chem.libretexts.org
6.9 Electron Configurations & the Periodic Table Chemistry LibreTexts Electron Configuration For Aluminum Atomic Number 13 Is Atomic mass, electron configurations, charges, and more. Access detailed info on all elements: Get a free hd image of the periodic. Since the 1s orbital can hold only two electrons the next two enter the 2s orbital. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are. Electron Configuration For Aluminum Atomic Number 13 Is.
From mavink.com
Aluminum Shell Diagram Electron Configuration For Aluminum Atomic Number 13 Is The atomic number of aluminum is 13. Since the 1s orbital can hold only two electrons the next two enter the 2s orbital. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. The element with atomic number 13 is aluminum. Aluminum’s atomic number is 13,. Electron Configuration For Aluminum Atomic Number 13 Is.
From www.sciencephoto.com
Aluminum, atomic structure Stock Image C018/3694 Science Photo Electron Configuration For Aluminum Atomic Number 13 Is To write the electron configuration for aluminum, the first two electrons enter the 1s orbital. A neutral atom of aluminum has 13 protons and 13 electrons. Atomic mass, electron configurations, charges, and more. Aluminum’s atomic number is 13, which means its atom has thirteen electrons around its nucleus. In order to write the aluminium electron configuration we first need to. Electron Configuration For Aluminum Atomic Number 13 Is.
From slideplayer.com
H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Electron Configuration For Aluminum Atomic Number 13 Is Get a free hd image of the periodic. To find the electron configuration of an atom, you first need to know the number of electrons that it has. The atomic number of aluminum is 13. Access detailed info on all elements: Since the 1s orbital can hold only two electrons the next two enter the 2s orbital. Aluminum’s atomic number. Electron Configuration For Aluminum Atomic Number 13 Is.
From www.youtube.com
Aluminum Ground State Electron Configuration YouTube Electron Configuration For Aluminum Atomic Number 13 Is Aluminum’s atomic number is 13, which means its atom has thirteen electrons around its nucleus. The element with atomic number 13 is aluminum. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. Atomic mass, electron configurations, charges, and more. The atomic number gives us the. Electron Configuration For Aluminum Atomic Number 13 Is.
From ar.inspiredpencil.com
Aluminium Atomic Structure Electron Configuration For Aluminum Atomic Number 13 Is The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. Get a free hd image of the periodic. To find the electron configuration of an atom, you first need to know the number of electrons that it has. To write the electron configuration for aluminum, the first two electrons enter the. Electron Configuration For Aluminum Atomic Number 13 Is.
From thenationstime.com
The Electron Configuration Of Aluminum Electron Configuration For Aluminum Atomic Number 13 Is The atomic number gives us the number of protons in an atom of aluminum. Since the 1s orbital can hold only two electrons the next two enter the 2s orbital. View rotating bohr models for all 118 elements. Access detailed info on all elements: The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2. Electron Configuration For Aluminum Atomic Number 13 Is.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Al Aluminium Element Information Facts, Properties, Trends, Uses and Electron Configuration For Aluminum Atomic Number 13 Is The atomic number gives us the number of protons in an atom of aluminum. Get a free hd image of the periodic. The electron configuration for aluminum (al), which has an atomic number of 13, is 1s2 2s2 2p6 3s2 3p1. Atomic mass, electron configurations, charges, and more. The element with atomic number 13 is aluminum. Access detailed info on. Electron Configuration For Aluminum Atomic Number 13 Is.
From chem.libretexts.org
Electronic Configurations Intro Chemistry LibreTexts Electron Configuration For Aluminum Atomic Number 13 Is The atomic number gives us the number of protons in an atom of aluminum. Aluminum’s atomic number is 13, which means its atom has thirteen electrons around its nucleus. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. A neutral atom of aluminum has 13. Electron Configuration For Aluminum Atomic Number 13 Is.