Medical Device Variant Definition . This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. Food and drug administration issued guidance for medical product developers, specifically covering. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. Variants are not completely different systems, but a single medical device with different configurations.
from microbenotes.com
The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. Food and drug administration issued guidance for medical product developers, specifically covering. This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. Variants are not completely different systems, but a single medical device with different configurations.
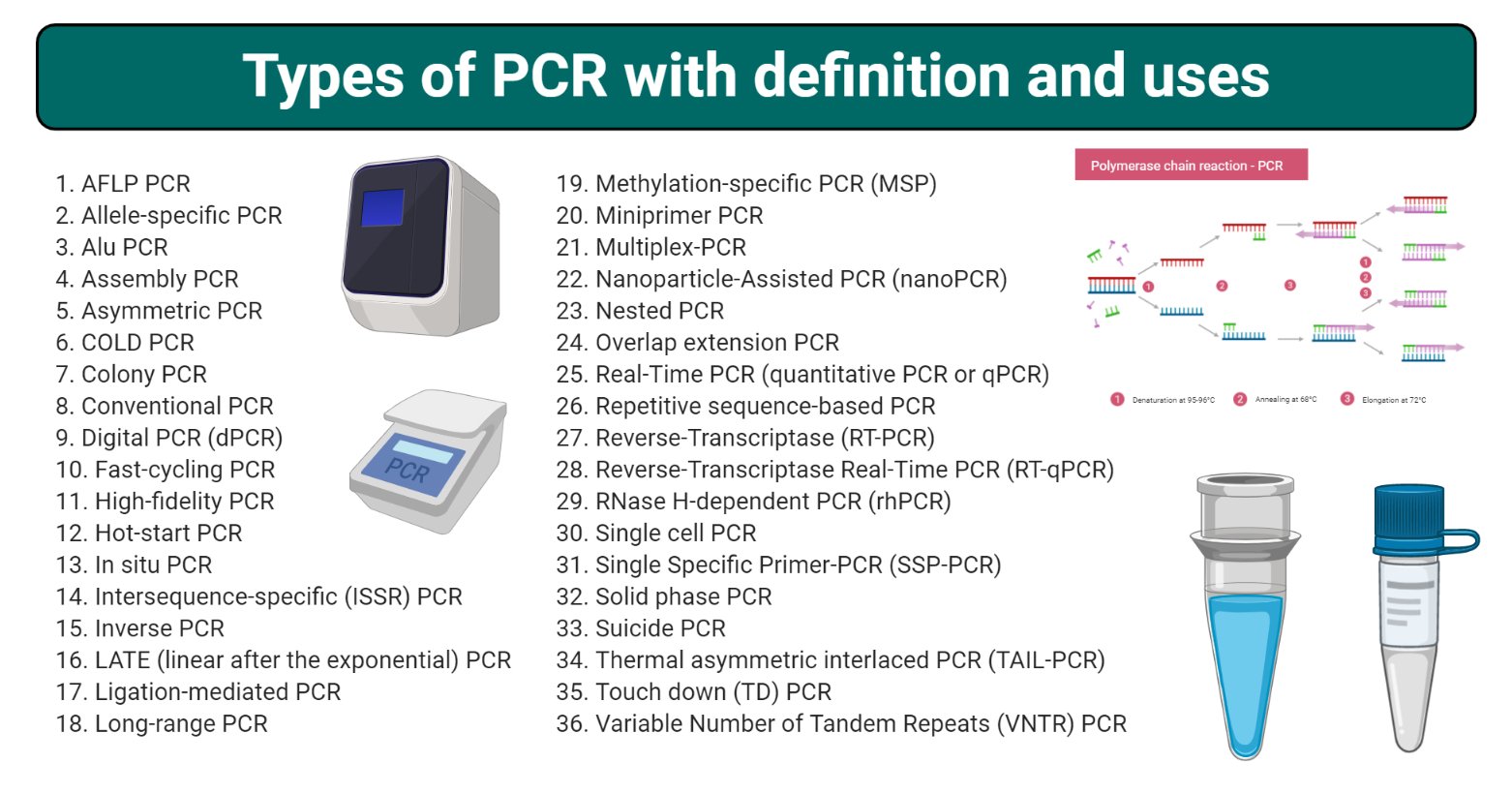
37 Types of PCR with Definition, Principle, and Uses
Medical Device Variant Definition Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. Variants are not completely different systems, but a single medical device with different configurations. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. Food and drug administration issued guidance for medical product developers, specifically covering. This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public.
From 3.11.12.166
HOW USERS DISTINGUISH BETWEEN SELFINJECTION DEVICE PLATFORM VARIANTS Medical Device Variant Definition Food and drug administration issued guidance for medical product developers, specifically covering. Variants are not completely different systems, but a single medical device with different configurations. This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity. Medical Device Variant Definition.
From www.medicalexpo.com
Medical software CT Stroke Beijing Infervision Technology Co. Ltd Medical Device Variant Definition This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. Food and drug administration issued guidance for medical product developers, specifically covering. Provides. Medical Device Variant Definition.
From accugenomics.com
Copy Number Variants Accugenomics Medical Device Variant Definition This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. Variants are not completely different systems, but a single medical device with different configurations. Food and drug administration issued guidance for medical. Medical Device Variant Definition.
From www.cell.com
Challenges and opportunities associated with rarevariant Medical Device Variant Definition The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. Variants are not completely different systems, but a single medical device with different configurations. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how. Medical Device Variant Definition.
From www.medicaldevice-network.com
Seegene launches new test to identify SARSCoV2 variants Medical Device Variant Definition Variants are not completely different systems, but a single medical device with different configurations. This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. This. Medical Device Variant Definition.
From www.pannam.com
Ultimate Guide to Medical Device Design and Development Pannam Medical Device Variant Definition This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. Variants are not completely different. Medical Device Variant Definition.
From www.slideshare.net
Medical devices rules 2015 (summary) Medical Device Variant Definition Food and drug administration issued guidance for medical product developers, specifically covering. This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. The enactment of the cares act at the height of the initial. Medical Device Variant Definition.
From www.medicaldevice-network.com
Hemex upgrades Gazelle Hb Variant to monitor hydroxyurea therapy Medical Device Variant Definition This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. Food and drug administration issued guidance for medical product developers, specifically covering. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. The enactment of the cares act at the height of the initial. Medical Device Variant Definition.
From compbio.berkeley.edu
VarantAn Open source tool for variant annotation Medical Device Variant Definition This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device. Medical Device Variant Definition.
From erpnext.com
Open Source Medical Device Manufacturing ERP Medical Device Variant Definition Food and drug administration issued guidance for medical product developers, specifically covering. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. Variants are not completely different systems, but a single medical. Medical Device Variant Definition.
From www.labmed.theclinics.com
The Evolution of Constitutional Sequence Variant Interpretation Medical Device Variant Definition Variants are not completely different systems, but a single medical device with different configurations. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. Provides. Medical Device Variant Definition.
From askthenurseexpert.com
A Deep Dive Into Covid19 Variants What We Know So Far Ask The Nurse Medical Device Variant Definition Variants are not completely different systems, but a single medical device with different configurations. Food and drug administration issued guidance for medical product developers, specifically covering. This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. The enactment of the cares act at the height of the initial pandemic response gave. Medical Device Variant Definition.
From www.unmc.edu
What to Know About the New Covid Variants The Transmission Medical Device Variant Definition This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. Food and drug administration issued guidance for medical product developers, specifically covering. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. Variants are not completely different systems, but a single medical. Medical Device Variant Definition.
From bmcmedinformdecismak.biomedcentral.com
Variant information systems for precision oncology BMC Medical Medical Device Variant Definition Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. This document seeks to provide guidance for clinical data providing sufficient clinical evidence. Medical Device Variant Definition.
From fastlabcorporation.com
Understanding the Latest COVID19 Variants A Comprehensive Overview Medical Device Variant Definition This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. Food and drug administration issued guidance for medical product developers, specifically covering. This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. Variants are not completely different systems, but a single medical device with. Medical Device Variant Definition.
From www.frontiersin.org
Frontiers Evaluation of MicrochipBased PointOfCare Device “Gazelle Medical Device Variant Definition Food and drug administration issued guidance for medical product developers, specifically covering. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. This document seeks. Medical Device Variant Definition.
From www.medicaldevice-network.com
PerkinElmer introduces new test kit to detect Covid19 variants Medical Device Variant Definition Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. Variants are not completely different. Medical Device Variant Definition.
From meddev-info.blogspot.com
Medical Device Regulation Basics IVDR Technical Documentation Medical Device Variant Definition Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. Variants are not completely different systems, but a single medical device with different. Medical Device Variant Definition.
From www.rimsys.io
De Novo classification process a beginner's guide Medical Device Variant Definition Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. Food and drug administration issued guidance for medical product developers, specifically covering. This article provides a primer on medical device regulation of. Medical Device Variant Definition.
From www.pulsus.com
Assessment of three patients with major vascular variations in head and Medical Device Variant Definition Food and drug administration issued guidance for medical product developers, specifically covering. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. Variants are not completely different systems, but a single medical. Medical Device Variant Definition.
From www.researchgate.net
(PDF) How to prevent medication errors A multidimensional scaling Medical Device Variant Definition This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. Food and drug administration issued guidance for medical product developers, specifically covering. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. Variants are not completely different systems, but a single medical device with. Medical Device Variant Definition.
From mdbio.com
Delta Variant Covid Test Kit Accurate and Reliable Medical Device Variant Definition This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. Food and drug administration issued guidance for medical product developers, specifically covering. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. Provides. Medical Device Variant Definition.
From courses.lumenlearning.com
Sources of Variation Boundless Anatomy and Physiology Medical Device Variant Definition The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. Food and drug administration issued guidance for medical product developers, specifically covering. This. Medical Device Variant Definition.
From microbenotes.com
37 Types of PCR with Definition, Principle, and Uses Medical Device Variant Definition The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. Food and drug administration issued guidance for medical product developers, specifically covering. This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. This. Medical Device Variant Definition.
From www.knowllence.com
Managing medical device variants our MD software Medical Device Variant Definition Variants are not completely different systems, but a single medical device with different configurations. Food and drug administration issued guidance for medical product developers, specifically covering. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. This document seeks to provide guidance. Medical Device Variant Definition.
From bantoo.app
Bantoo Modern, DIY ERP For Medical Device Manufacturers Medical Device Variant Definition Food and drug administration issued guidance for medical product developers, specifically covering. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. This. Medical Device Variant Definition.
From cliniexperts.com
Medical Device Grouping as per MDR 2017 CliniExperts CliniExperts Medical Device Variant Definition The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. Food and drug administration issued guidance for medical product developers, specifically covering. Variants are not completely different systems, but a single medical device with different configurations. This article provides a primer on. Medical Device Variant Definition.
From definitionghw.blogspot.com
What Is The Definition Of Variant DEFINITION GHW Medical Device Variant Definition Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. This article provides a primer on medical device regulation of software that interprets. Medical Device Variant Definition.
From journals.sagepub.com
New Coronavirus Variants are Creating More Challenges to Global Medical Device Variant Definition This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device. Medical Device Variant Definition.
From askthenurseexpert.com
A Deep Dive Into Covid19 Variants What We Know So Far Ask The Nurse Medical Device Variant Definition Food and drug administration issued guidance for medical product developers, specifically covering. This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. Variants are not completely different systems, but a single medical device with different configurations. Provides additional information on what constitutes an acceptable variant for a medical device, and gives. Medical Device Variant Definition.
From rosalyndwmisty.pages.dev
Covid Variants Names List 2024 Orsa Trenna Medical Device Variant Definition This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. Food and drug administration issued guidance for medical product developers, specifically covering. This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. The enactment of the cares act at the height of the initial. Medical Device Variant Definition.
From intermountainhealthcare.org
Understanding the New COVID19 Variants, and How to Fight Them Medical Device Variant Definition Variants are not completely different systems, but a single medical device with different configurations. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the. Medical Device Variant Definition.
From www.alamy.com
Serology antibody testing,rapid diagnostic test kit cassette,express Medical Device Variant Definition This document seeks to provide guidance for clinical data providing sufficient clinical evidence necessary to demonstrate conformity with the. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on how to. Food and drug administration issued guidance for medical product developers, specifically covering. Variants are not completely different systems, but a single medical. Medical Device Variant Definition.
From definitionghw.blogspot.com
What Is The Definition Of Variant DEFINITION GHW Medical Device Variant Definition This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. Variants are not completely different systems, but a single medical device with different configurations. Food and drug administration issued guidance for medical product developers, specifically covering. Provides additional information on what constitutes an acceptable variant for a medical device, and gives instruction on. Medical Device Variant Definition.
From www.researchgate.net
Examples of LGE sequence variants in device patients. Comparison of Medical Device Variant Definition This article provides a primer on medical device regulation of software that interprets and exchanges genomic data. The enactment of the cares act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public. Food and drug administration issued guidance for medical product developers, specifically covering. Variants are not. Medical Device Variant Definition.