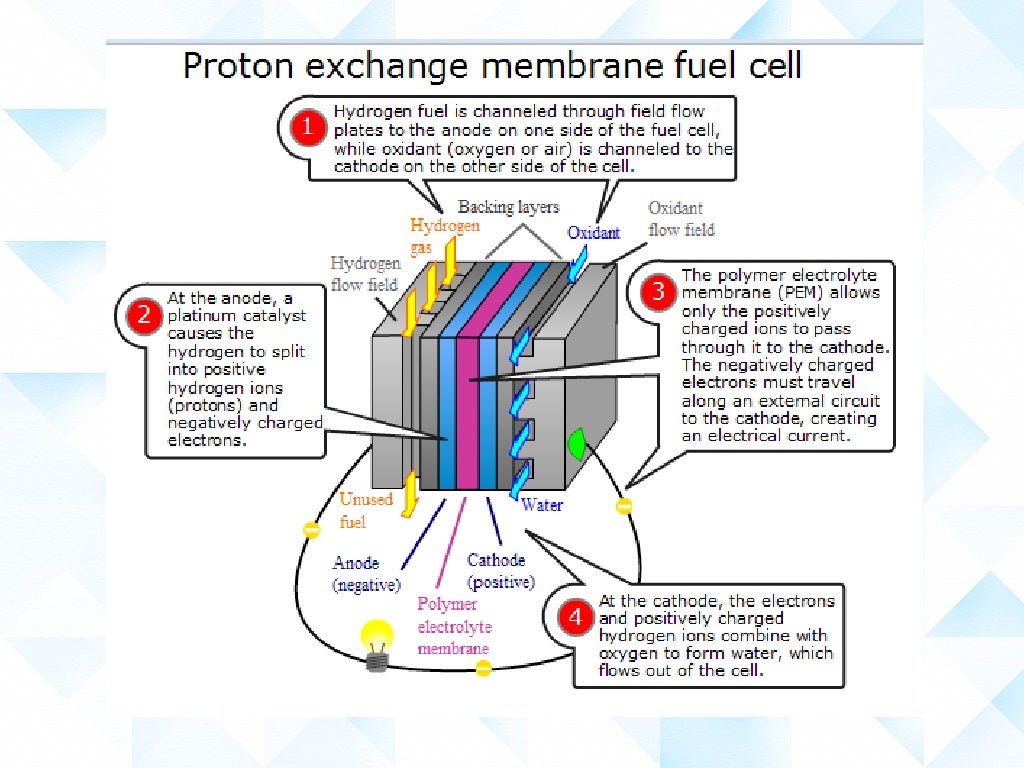
Hydrogen Fuel Cells A Level Chemistry . The most common type of fuel cell is the hydrogen fuel cell , which uses a continuous supply of hydrogen and oxygen from the air to. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. This is done when an electrochemical reaction takes place between hydrogen. Fuel cells have an important advantage over all other devices which burn fuel to obtain useful energy: What are the reactions that take place at the 2 electrons in an alkaline hydrogen fuel cell? Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Different electrolytes and fuels can be used to set up different types of fuel cells. Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g.
from www.slideshare.net
Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Fuel cells have an important advantage over all other devices which burn fuel to obtain useful energy: What are the reactions that take place at the 2 electrons in an alkaline hydrogen fuel cell? Different electrolytes and fuels can be used to set up different types of fuel cells. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. The most common type of fuel cell is the hydrogen fuel cell , which uses a continuous supply of hydrogen and oxygen from the air to. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. This is done when an electrochemical reaction takes place between hydrogen. Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g.
Hydrogen fuel cells
Hydrogen Fuel Cells A Level Chemistry The most common type of fuel cell is the hydrogen fuel cell , which uses a continuous supply of hydrogen and oxygen from the air to. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. This is done when an electrochemical reaction takes place between hydrogen. Different electrolytes and fuels can be used to set up different types of fuel cells. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. What are the reactions that take place at the 2 electrons in an alkaline hydrogen fuel cell? Fuel cells have an important advantage over all other devices which burn fuel to obtain useful energy: The most common type of fuel cell is the hydrogen fuel cell , which uses a continuous supply of hydrogen and oxygen from the air to. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g.
From www.branduk.net
Hydrogen fuel cells are the longterm solution but what about today Hydrogen Fuel Cells A Level Chemistry Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. Different electrolytes and fuels can be used to set up different types of fuel cells. This is done when an electrochemical reaction takes place between hydrogen. The most common type of fuel cell is the hydrogen fuel cell , which uses a continuous. Hydrogen Fuel Cells A Level Chemistry.
From www.africanmining.co.za
Platinum the winner in hydrogen fuel cells African Mining Online Hydrogen Fuel Cells A Level Chemistry This is done when an electrochemical reaction takes place between hydrogen. Different electrolytes and fuels can be used to set up different types of fuel cells. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air). Hydrogen Fuel Cells A Level Chemistry.
From nwpublicmedia.typepad.com
NWPR Energy Series Part Three Hydrogen Fuel Cells Our Sustainable Hydrogen Fuel Cells A Level Chemistry Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. What are the reactions that take place at the 2 electrons in an alkaline hydrogen fuel cell? Different electrolytes and fuels can. Hydrogen Fuel Cells A Level Chemistry.
From w.media
Microsoft Data Center to Host Demonstration Project Using Hydrogen Fuel Hydrogen Fuel Cells A Level Chemistry Fuel cells have an important advantage over all other devices which burn fuel to obtain useful energy: Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Hydrogen—oxygen fuel cells can operate. Hydrogen Fuel Cells A Level Chemistry.
From mavink.com
Hydrogen Oxygen Fuel Cell Diagram Hydrogen Fuel Cells A Level Chemistry Fuel cells have an important advantage over all other devices which burn fuel to obtain useful energy: Different electrolytes and fuels can be used to set up different types of fuel cells. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. What are the reactions that take place. Hydrogen Fuel Cells A Level Chemistry.
From www.mdpi.com
Energies Free FullText Developments in Hydrogen Fuel Cells Hydrogen Fuel Cells A Level Chemistry Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. This is done when an electrochemical reaction takes place between hydrogen. Fuel cells have an important advantage over all other devices which burn fuel to obtain useful energy: Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen. Hydrogen Fuel Cells A Level Chemistry.
From inhabitat.com
Scientists create innovative hydrogen fuel "nanoreactor" that could Hydrogen Fuel Cells A Level Chemistry Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. Fuel cells have an important advantage over all other devices which burn fuel to obtain useful energy: Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. This is done when an. Hydrogen Fuel Cells A Level Chemistry.
From pressbooks.openedmb.ca
Batteries and Fuel Cells Chemistry and the Environment Hydrogen Fuel Cells A Level Chemistry The most common type of fuel cell is the hydrogen fuel cell , which uses a continuous supply of hydrogen and oxygen from the air to. This is done when an electrochemical reaction takes place between hydrogen. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. What are the reactions that take. Hydrogen Fuel Cells A Level Chemistry.
From climatebiz.com
How To Build A DIY Hydrogen Fuel Cell Hydrogen Fuel Cells A Level Chemistry This is done when an electrochemical reaction takes place between hydrogen. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. What are the reactions that take place at the 2 electrons in an alkaline hydrogen fuel cell? Fuel cells have an important advantage over all other devices which. Hydrogen Fuel Cells A Level Chemistry.
From www.thoughtco.com
Hydrogen Fuel Cells Innovation for the 21st Century Hydrogen Fuel Cells A Level Chemistry This is done when an electrochemical reaction takes place between hydrogen. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Fuel cells have an important advantage over all other devices which. Hydrogen Fuel Cells A Level Chemistry.
From climatebiz.com
How Do Hydrogen Fuel Cells Work? Hydrogen Fuel Cells A Level Chemistry Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. This is done when an electrochemical reaction takes place between hydrogen. Fuel cells have an important advantage over all other devices which burn fuel to obtain useful energy: Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions. Hydrogen Fuel Cells A Level Chemistry.
From paylesspower.com
Advantages & Disadvantages of Hydrogen Fuel Cells Payless Power Hydrogen Fuel Cells A Level Chemistry Different electrolytes and fuels can be used to set up different types of fuel cells. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. This is done when an electrochemical reaction takes place between hydrogen. The most common type of fuel cell is the hydrogen fuel cell ,. Hydrogen Fuel Cells A Level Chemistry.
From www.researchgate.net
Schematic diagram of hydrogen fuel cell Download Scientific Diagram Hydrogen Fuel Cells A Level Chemistry Different electrolytes and fuels can be used to set up different types of fuel cells. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Hydrogen—oxygen fuel cells can operate in acidic. Hydrogen Fuel Cells A Level Chemistry.
From automotiveseo.org
Pros and Cons of Hydrogen Fuel Cells Hydrogen Fuel Cells A Level Chemistry Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. Different electrolytes and fuels can be used to set up different types of fuel cells. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. Fuel cells have an important advantage over. Hydrogen Fuel Cells A Level Chemistry.
From www.hydrogenfuelnews.com
Shell And HyAxiom Sign Agreement To Test Hydrogen Fuel Cells On A Large Hydrogen Fuel Cells A Level Chemistry This is done when an electrochemical reaction takes place between hydrogen. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Different electrolytes and fuels can be used to set up different types of fuel cells. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial. Hydrogen Fuel Cells A Level Chemistry.
From www.slideshare.net
Hydrogen fuel cells Hydrogen Fuel Cells A Level Chemistry This is done when an electrochemical reaction takes place between hydrogen. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. What are the reactions that take place at the 2 electrons in an alkaline hydrogen fuel cell? The most common type of fuel cell is the hydrogen fuel. Hydrogen Fuel Cells A Level Chemistry.
From www.aeologic.com
Role of Hydrogen Fuel Cells in Global Energy System Aeologic Blog Hydrogen Fuel Cells A Level Chemistry Different electrolytes and fuels can be used to set up different types of fuel cells. What are the reactions that take place at the 2 electrons in an alkaline hydrogen fuel cell? The most common type of fuel cell is the hydrogen fuel cell , which uses a continuous supply of hydrogen and oxygen from the air to. Hydrogen—oxygen fuel. Hydrogen Fuel Cells A Level Chemistry.
From www.researchgate.net
Schematic illustration of a hydrogen fuel cell. Download Scientific Hydrogen Fuel Cells A Level Chemistry Fuel cells have an important advantage over all other devices which burn fuel to obtain useful energy: Different electrolytes and fuels can be used to set up different types of fuel cells. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. Fuel cells are the type of electrochemical. Hydrogen Fuel Cells A Level Chemistry.
From www.innovationnewsnetwork.com
EU grants €12.4m to STRING for crossborder hydrogen fuel stations Hydrogen Fuel Cells A Level Chemistry Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. What. Hydrogen Fuel Cells A Level Chemistry.
From www.fchea.org
Cummins H2 Day Post Five key questions about the next frontier Hydrogen Fuel Cells A Level Chemistry Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. This is done when an. Hydrogen Fuel Cells A Level Chemistry.
From www.youtube.com
AQA GCSE Chemistry P1 Hydrogen Fuel Cells YouTube Hydrogen Fuel Cells A Level Chemistry This is done when an electrochemical reaction takes place between hydrogen. Fuel cells have an important advantage over all other devices which burn fuel to obtain useful energy: Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as. Hydrogen Fuel Cells A Level Chemistry.
From azgreenmagazine.com
What Are the Pros and Cons of Hydrogen Fuel Cells? Hydrogen Fuel Cells A Level Chemistry Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. Different electrolytes and fuels can be used to set up different types of fuel cells. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. The most common type of fuel cell. Hydrogen Fuel Cells A Level Chemistry.
From www.researchgate.net
e Schematic view of a typical hydrogen PEM fuel cell and its components Hydrogen Fuel Cells A Level Chemistry Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. This is done when an. Hydrogen Fuel Cells A Level Chemistry.
From www.setra.com
What is a Hydrogen Fuel Cell? Hydrogen Fuel Cells A Level Chemistry Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Different electrolytes and fuels can. Hydrogen Fuel Cells A Level Chemistry.
From www.narayannepal.com.np
Electrochemistry Class 12 Chemistry Notes NEB Best NEB Notes Hydrogen Fuel Cells A Level Chemistry The most common type of fuel cell is the hydrogen fuel cell , which uses a continuous supply of hydrogen and oxygen from the air to. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. What are the reactions that take place at the 2 electrons in an. Hydrogen Fuel Cells A Level Chemistry.
From news.microsoft.com
Hydrogen fuel cells could provide emission free backup power at Hydrogen Fuel Cells A Level Chemistry Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. What are the reactions that take place at the 2 electrons in an alkaline hydrogen fuel cell? Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. The most common type of. Hydrogen Fuel Cells A Level Chemistry.
From www.hydrogenfuelnews.com
UAM Hydrogen Fuel Cells In Hanwha Aerospace Future H2 News Hydrogen Fuel Cells A Level Chemistry This is done when an electrochemical reaction takes place between hydrogen. Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Fuel cells have an important advantage over all other devices which. Hydrogen Fuel Cells A Level Chemistry.
From revisechemistry.uk
Chemical Cells and Fuel Cells AQA C5 revisechemistry.uk Hydrogen Fuel Cells A Level Chemistry This is done when an electrochemical reaction takes place between hydrogen. Different electrolytes and fuels can be used to set up different types of fuel cells. Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. What are the reactions that take place at the 2 electrons in an. Hydrogen Fuel Cells A Level Chemistry.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars Hydrogen Fuel Cells A Level Chemistry Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. This is done when an electrochemical reaction takes place between hydrogen. The most common type of fuel cell is the hydrogen fuel cell , which uses a continuous supply of hydrogen and oxygen from the air to. What are the reactions that take. Hydrogen Fuel Cells A Level Chemistry.
From leehamnews.com
Bjorn’s Corner The challenges of hydrogen. Part 23. Hydrogen fuel Hydrogen Fuel Cells A Level Chemistry Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. Different electrolytes and fuels can be used to set up different types of fuel cells. The most common type of fuel cell is the hydrogen fuel cell , which uses a continuous supply of hydrogen and oxygen from the air to. This is. Hydrogen Fuel Cells A Level Chemistry.
From acapmag.com.au
Titan Hydrogen progressing its innovative hydrogen fuel cell technology Hydrogen Fuel Cells A Level Chemistry The most common type of fuel cell is the hydrogen fuel cell , which uses a continuous supply of hydrogen and oxygen from the air to. Fuel cells have an important advantage over all other devices which burn fuel to obtain useful energy: Different electrolytes and fuels can be used to set up different types of fuel cells. Fuel cells. Hydrogen Fuel Cells A Level Chemistry.
From www.forbes.com
It's Time For Elon Musk To Admit The Significance Of Hydrogen Fuel Cells Hydrogen Fuel Cells A Level Chemistry What are the reactions that take place at the 2 electrons in an alkaline hydrogen fuel cell? Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. Fuel cells have an important advantage over all other devices which burn fuel to obtain useful energy: This is done when an. Hydrogen Fuel Cells A Level Chemistry.
From www.visualcapitalist.com
How Hydrogen and Fuel Cells Can Help Drive the Clean Energy Transition Hydrogen Fuel Cells A Level Chemistry Hydrogen—oxygen fuel cells can operate in acidic or in alkaline conditions but commercial cells use porous platinum electrodes in contact with. Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g. Typically, in a hydrogen fuel cell, hydrogen gas (h2) acts as the anode, while oxygen gas (o2) acts as the cathode. Fuel. Hydrogen Fuel Cells A Level Chemistry.
From www.chfca.ca
About Fuel Cells CHFCA Hydrogen Fuel Cells A Level Chemistry What are the reactions that take place at the 2 electrons in an alkaline hydrogen fuel cell? The most common type of fuel cell is the hydrogen fuel cell , which uses a continuous supply of hydrogen and oxygen from the air to. Fuel cells generate electricity from an electrochemical reaction in which oxygen (from air) and a fuel (e.g.. Hydrogen Fuel Cells A Level Chemistry.
From www.air101.co.uk
Air101 Hydrogen fuel cells, explained Hydrogen Fuel Cells A Level Chemistry The most common type of fuel cell is the hydrogen fuel cell , which uses a continuous supply of hydrogen and oxygen from the air to. Fuel cells are the type of electrochemical cells that convert the chemical energy of fuel into electricity. This is done when an electrochemical reaction takes place between hydrogen. Different electrolytes and fuels can be. Hydrogen Fuel Cells A Level Chemistry.