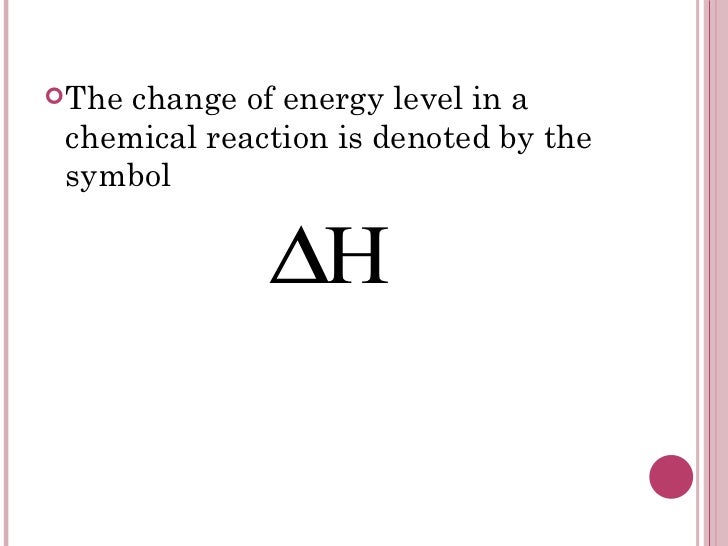
Heating Symbol In Chemistry . For chemical reactions, it's usually difference between energy that reactants already have and energy needed for reaction. [4] heat released by a system into its surroundings. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. Check out the energy diagram. The symbol q for heat was introduced by rudolf clausius and macquorn rankine in c. What is heat (enthalpy) of reaction. It is the change in internal energy that produces heat plus work. It has units of energy per mole reaction (rxn). A negative \(δh\) means that heat flows from a system to its surroundings; Learn its equation, unit, and symbol, along with a few examples. The heat for a reaction is given the symbol \(\delta h_{\rm rxn}^\circ \). To measure the energy changes that occur in chemical. Per mole reaction refers to the balanced equation. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs. A positive δh means that heat flows into a.
from www.slideshare.net
For chemical reactions, it's usually difference between energy that reactants already have and energy needed for reaction. [4] heat released by a system into its surroundings. The heat for a reaction is given the symbol \(\delta h_{\rm rxn}^\circ \). The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs. A positive δh means that heat flows into a. What is heat (enthalpy) of reaction. It has units of energy per mole reaction (rxn). Check out the energy diagram. It is the change in internal energy that produces heat plus work. To measure the energy changes that occur in chemical.
Heat changes in chemical reactions
Heating Symbol In Chemistry The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs. A negative \(δh\) means that heat flows from a system to its surroundings; It is the change in internal energy that produces heat plus work. To measure the energy changes that occur in chemical. What is heat (enthalpy) of reaction. For chemical reactions, it's usually difference between energy that reactants already have and energy needed for reaction. Per mole reaction refers to the balanced equation. A positive δh means that heat flows into a. It has units of energy per mole reaction (rxn). The heat for a reaction is given the symbol \(\delta h_{\rm rxn}^\circ \). Check out the energy diagram. [4] heat released by a system into its surroundings. Learn its equation, unit, and symbol, along with a few examples. The symbol q for heat was introduced by rudolf clausius and macquorn rankine in c. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs.
From owlcation.com
Chemical Reactions and Chemical Equations Owlcation Heating Symbol In Chemistry Per mole reaction refers to the balanced equation. Check out the energy diagram. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs. For chemical reactions, it's usually difference between energy that reactants already have and energy needed for reaction. The symbol q for heat was introduced by. Heating Symbol In Chemistry.
From enggcyclopedia.com
P&ID Symbols EnggCyclopedia Heating Symbol In Chemistry A negative \(δh\) means that heat flows from a system to its surroundings; Per mole reaction refers to the balanced equation. It has units of energy per mole reaction (rxn). It is the change in internal energy that produces heat plus work. Check out the energy diagram. Learn its equation, unit, and symbol, along with a few examples. The heat. Heating Symbol In Chemistry.
From studylib.net
Heat Equation Heating Symbol In Chemistry [4] heat released by a system into its surroundings. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. To measure the energy changes that occur in chemical. The symbol q for heat was introduced by rudolf clausius and macquorn rankine in. Heating Symbol In Chemistry.
From www.alamy.com
Heating Element High Resolution Stock Photography and Images Alamy Heating Symbol In Chemistry Learn its equation, unit, and symbol, along with a few examples. To measure the energy changes that occur in chemical. A positive δh means that heat flows into a. [4] heat released by a system into its surroundings. It is the change in internal energy that produces heat plus work. What is heat (enthalpy) of reaction. Check out the energy. Heating Symbol In Chemistry.
From www.slideserve.com
PPT Thermochemistry The heat energy of chemical reactions PowerPoint Heating Symbol In Chemistry A positive δh means that heat flows into a. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. To measure the energy changes that occur in chemical. For chemical reactions, it's usually difference between energy that reactants already have and energy. Heating Symbol In Chemistry.
From www.edrawmax.com
Heat Exchangers P&ID Symbols EdrawMax Templates Heating Symbol In Chemistry The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs. [4] heat released by a system into its surroundings. Learn its equation, unit, and symbol, along with a few examples. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in. Heating Symbol In Chemistry.
From mavink.com
Chemical Reaction Symbols Heating Symbol In Chemistry The symbol q for heat was introduced by rudolf clausius and macquorn rankine in c. It is the change in internal energy that produces heat plus work. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. To measure the energy changes. Heating Symbol In Chemistry.
From www.clker.com
Heat Symbol Clip Art at vector clip art online, royalty Heating Symbol In Chemistry To measure the energy changes that occur in chemical. Learn its equation, unit, and symbol, along with a few examples. The symbol q for heat was introduced by rudolf clausius and macquorn rankine in c. For chemical reactions, it's usually difference between energy that reactants already have and energy needed for reaction. A negative \(δh\) means that heat flows from. Heating Symbol In Chemistry.
From stock.adobe.com
heat sign, heat wave of steam, superheated steam symbol Stock Vector Heating Symbol In Chemistry To measure the energy changes that occur in chemical. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs. The. Heating Symbol In Chemistry.
From www.youtube.com
Symbols for Chemical reactions YouTube Heating Symbol In Chemistry To measure the energy changes that occur in chemical. It is the change in internal energy that produces heat plus work. It has units of energy per mole reaction (rxn). The heat for a reaction is given the symbol \(\delta h_{\rm rxn}^\circ \). What is heat (enthalpy) of reaction. A negative \(δh\) means that heat flows from a system to. Heating Symbol In Chemistry.
From www.youtube.com
Specific Heat Equation Stated Clearly YouTube Heating Symbol In Chemistry Check out the energy diagram. It has units of energy per mole reaction (rxn). The symbol q for heat was introduced by rudolf clausius and macquorn rankine in c. To measure the energy changes that occur in chemical. It is the change in internal energy that produces heat plus work. A negative \(δh\) means that heat flows from a system. Heating Symbol In Chemistry.
From www.clipartkey.com
Heat Clipart Heating Thermometer Heat Icon Png , Free Transparent Heating Symbol In Chemistry It is the change in internal energy that produces heat plus work. What is heat (enthalpy) of reaction. The heat for a reaction is given the symbol \(\delta h_{\rm rxn}^\circ \). [4] heat released by a system into its surroundings. To measure the energy changes that occur in chemical. The symbol q for heat was introduced by rudolf clausius and. Heating Symbol In Chemistry.
From engineeringtraining.tpub.com
APPENDIX IV CONTINUED 14069_625 Heating Symbol In Chemistry Learn its equation, unit, and symbol, along with a few examples. It is the change in internal energy that produces heat plus work. It has units of energy per mole reaction (rxn). The symbol q for heat was introduced by rudolf clausius and macquorn rankine in c. Heat of reaction, the amount of heat that must be added or removed. Heating Symbol In Chemistry.
From www.slideserve.com
PPT Chapter 8 Chemical Equations and Reactions PowerPoint Heating Symbol In Chemistry Learn its equation, unit, and symbol, along with a few examples. What is heat (enthalpy) of reaction. Per mole reaction refers to the balanced equation. [4] heat released by a system into its surroundings. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs. A negative \(δh\) means. Heating Symbol In Chemistry.
From www.youtube.com
Symbols and Notations in Heat transfer YouTube Heating Symbol In Chemistry The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs. For chemical reactions, it's usually difference between energy that reactants already have and energy needed for reaction. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep. Heating Symbol In Chemistry.
From www.toppr.com
Specific Heat Formula Definition, Equations, Examples Heating Symbol In Chemistry It has units of energy per mole reaction (rxn). Learn its equation, unit, and symbol, along with a few examples. The heat for a reaction is given the symbol \(\delta h_{\rm rxn}^\circ \). [4] heat released by a system into its surroundings. A negative \(δh\) means that heat flows from a system to its surroundings; The heat of reaction (also. Heating Symbol In Chemistry.
From www.alamy.com
Underfloor heating element linear icon. Floor heating system. Thin line Heating Symbol In Chemistry Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. Learn its equation, unit, and symbol, along with a few examples. It is the change in internal energy that produces heat plus work. It has units of energy per mole reaction (rxn).. Heating Symbol In Chemistry.
From www.slideserve.com
PPT Heat in Chemical Reactions PowerPoint Presentation, free download Heating Symbol In Chemistry It has units of energy per mole reaction (rxn). For chemical reactions, it's usually difference between energy that reactants already have and energy needed for reaction. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs. Check out the energy diagram. The heat for a reaction is given. Heating Symbol In Chemistry.
From www.chegg.com
Solved Equipment Symbols Process Line Symbols Heat Heating Symbol In Chemistry A negative \(δh\) means that heat flows from a system to its surroundings; For chemical reactions, it's usually difference between energy that reactants already have and energy needed for reaction. Per mole reaction refers to the balanced equation. It is the change in internal energy that produces heat plus work. It has units of energy per mole reaction (rxn). A. Heating Symbol In Chemistry.
From secureservercdn.net
P&ID Symbols and Notation By Heating Symbol In Chemistry A negative \(δh\) means that heat flows from a system to its surroundings; For chemical reactions, it's usually difference between energy that reactants already have and energy needed for reaction. Learn its equation, unit, and symbol, along with a few examples. To measure the energy changes that occur in chemical. The symbol q for heat was introduced by rudolf clausius. Heating Symbol In Chemistry.
From www.lucidchart.com
P&ID Symbols and Notation Lucidchart Heating Symbol In Chemistry A positive δh means that heat flows into a. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs. The heat for a reaction is given the symbol \(\delta h_{\rm rxn}^\circ \). It is the change in internal energy that produces heat plus work. [4] heat released by. Heating Symbol In Chemistry.
From diagramdiagramdaniel77.z19.web.core.windows.net
Heating Element Schematic Symbol Heating Symbol In Chemistry What is heat (enthalpy) of reaction. To measure the energy changes that occur in chemical. Learn its equation, unit, and symbol, along with a few examples. A negative \(δh\) means that heat flows from a system to its surroundings; A positive δh means that heat flows into a. The symbol q for heat was introduced by rudolf clausius and macquorn. Heating Symbol In Chemistry.
From www.dreamstime.com
Heating Element Icon, Vector Illustration, Black Sign on Isolated Heating Symbol In Chemistry What is heat (enthalpy) of reaction. A negative \(δh\) means that heat flows from a system to its surroundings; Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. It has units of energy per mole reaction (rxn). Check out the energy. Heating Symbol In Chemistry.
From rillityworwayfqschematic.z21.web.core.windows.net
Heat Exchanger Schematic Symbol Heating Symbol In Chemistry Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. A negative \(δh\) means that heat flows from a system to its surroundings; The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical. Heating Symbol In Chemistry.
From mavink.com
Chemical Reaction Symbols Heating Symbol In Chemistry Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. [4] heat released by a system into its surroundings. Learn its equation, unit, and symbol, along with a few examples. A negative \(δh\) means that heat flows from a system to its. Heating Symbol In Chemistry.
From chemicaltweak.com
Learn P&ID Diagram Basics Symbols To Read P&ID Diagrams Easily Heating Symbol In Chemistry The symbol q for heat was introduced by rudolf clausius and macquorn rankine in c. What is heat (enthalpy) of reaction. A negative \(δh\) means that heat flows from a system to its surroundings; Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present. Heating Symbol In Chemistry.
From www.alamy.com
Heating glyph icons set. Electric and gas water heaters, heating boiler Heating Symbol In Chemistry It has units of energy per mole reaction (rxn). The heat for a reaction is given the symbol \(\delta h_{\rm rxn}^\circ \). Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. A positive δh means that heat flows into a. [4]. Heating Symbol In Chemistry.
From www.alamy.com
A vector illustration of Heat Temperature Wave Symbol Logo Icon Stock Heating Symbol In Chemistry The heat for a reaction is given the symbol \(\delta h_{\rm rxn}^\circ \). Learn its equation, unit, and symbol, along with a few examples. It is the change in internal energy that produces heat plus work. It has units of energy per mole reaction (rxn). To measure the energy changes that occur in chemical. [4] heat released by a system. Heating Symbol In Chemistry.
From www.showme.com
ShowMe safety symbols Heating Symbol In Chemistry It is the change in internal energy that produces heat plus work. What is heat (enthalpy) of reaction. To measure the energy changes that occur in chemical. [4] heat released by a system into its surroundings. For chemical reactions, it's usually difference between energy that reactants already have and energy needed for reaction. The heat for a reaction is given. Heating Symbol In Chemistry.
From www.slideshare.net
Heat changes in chemical reactions Heating Symbol In Chemistry A positive δh means that heat flows into a. Per mole reaction refers to the balanced equation. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs. What is heat (enthalpy) of reaction. Heat of reaction, the amount of heat that must be added or removed during a. Heating Symbol In Chemistry.
From www.edrawmax.com
Chemical Symbols and Meanings EdrawMax Online Heating Symbol In Chemistry Check out the energy diagram. [4] heat released by a system into its surroundings. A negative \(δh\) means that heat flows from a system to its surroundings; Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. Learn its equation, unit, and. Heating Symbol In Chemistry.
From www.alamy.com
A vector illustration of Heat Temperature Wave Symbol Logo Icon Stock Heating Symbol In Chemistry The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs. Check out the energy diagram. For chemical reactions, it's usually difference between energy that reactants already have and energy needed for reaction. It has units of energy per mole reaction (rxn). What is heat (enthalpy) of reaction. [4]. Heating Symbol In Chemistry.
From quizzlistreplevies.z13.web.core.windows.net
Heat Required For Phase Change Formula Heating Symbol In Chemistry A negative \(δh\) means that heat flows from a system to its surroundings; What is heat (enthalpy) of reaction. A positive δh means that heat flows into a. To measure the energy changes that occur in chemical. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of. Heating Symbol In Chemistry.
From www.slideserve.com
PPT Chemical Reactions and Equations PowerPoint Presentation ID5606806 Heating Symbol In Chemistry Learn its equation, unit, and symbol, along with a few examples. To measure the energy changes that occur in chemical. It is the change in internal energy that produces heat plus work. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs. Check out the energy diagram. A. Heating Symbol In Chemistry.
From www.edrawmax.com
P&ID Symbols and Meanings EdrawMax Online Heating Symbol In Chemistry For chemical reactions, it's usually difference between energy that reactants already have and energy needed for reaction. What is heat (enthalpy) of reaction. It has units of energy per mole reaction (rxn). To measure the energy changes that occur in chemical. Check out the energy diagram. A positive δh means that heat flows into a. A negative \(δh\) means that. Heating Symbol In Chemistry.