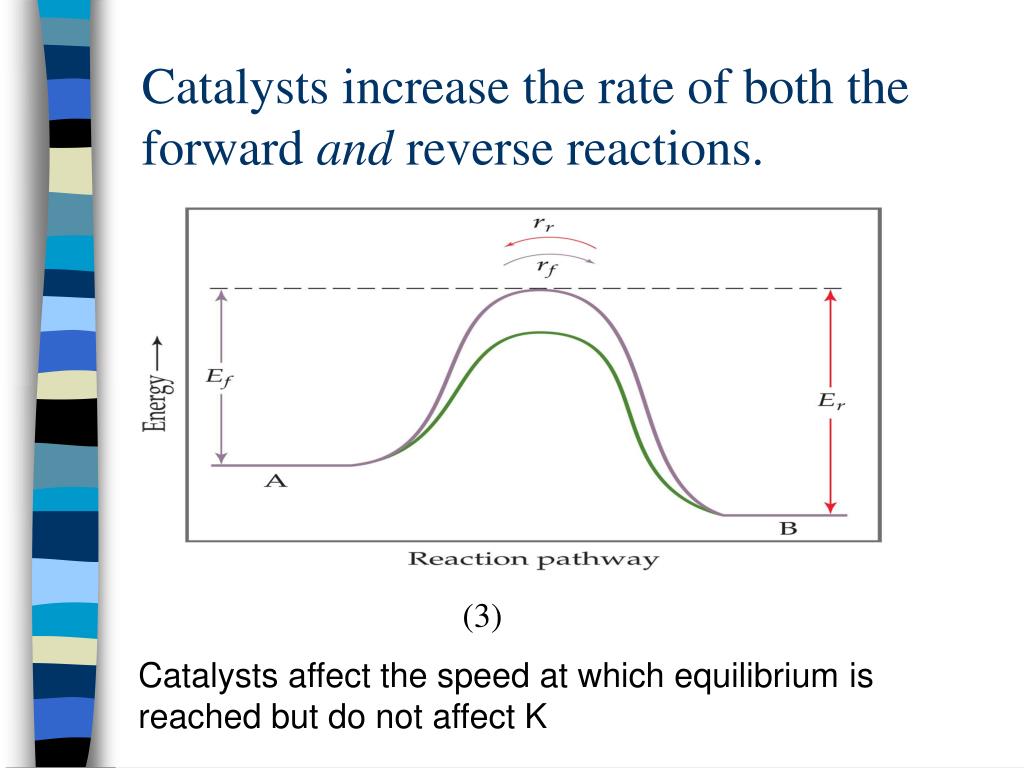
Why Don't Catalysts Affect Equilibrium . If the conditions of a. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Though this increase in reaction rate may cause a system to reach equilibrium more quickly (by speeding up the forward and. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward and backward. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). In such a reaction a catalyst speeds up both the forward and the backward reactions. Hence, although the system will reach dynamic. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates.
from www.slideserve.com
If the conditions of a. Hence, although the system will reach dynamic. Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward and backward. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. Though this increase in reaction rate may cause a system to reach equilibrium more quickly (by speeding up the forward and. In such a reaction a catalyst speeds up both the forward and the backward reactions.
PPT Chemical Equilibrium PowerPoint Presentation, free download ID
Why Don't Catalysts Affect Equilibrium Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. In such a reaction a catalyst speeds up both the forward and the backward reactions. Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward and backward. Though this increase in reaction rate may cause a system to reach equilibrium more quickly (by speeding up the forward and. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). If the conditions of a. Hence, although the system will reach dynamic. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition.
From hxeaspeqb.blob.core.windows.net
Why Do Catalysts Not Affect The Position Of Equilibrium at Erica Murphy Why Don't Catalysts Affect Equilibrium Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). In such a reaction a catalyst speeds up both the forward and the backward reactions. If the conditions of a. Catalysts do not affect the. Why Don't Catalysts Affect Equilibrium.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review Why Don't Catalysts Affect Equilibrium A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. Hence, although the system will reach dynamic. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and. Why Don't Catalysts Affect Equilibrium.
From slideplayer.com
Le Chatelier’s Principle and Equilibrium ppt download Why Don't Catalysts Affect Equilibrium Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. In such a reaction a catalyst speeds up both the forward and the backward reactions. Though this increase in reaction rate may cause a system to. Why Don't Catalysts Affect Equilibrium.
From www.slideshare.net
Intro to equilibrium abbrev alg Why Don't Catalysts Affect Equilibrium In such a reaction a catalyst speeds up both the forward and the backward reactions. Though this increase in reaction rate may cause a system to reach equilibrium more quickly (by speeding up the forward and. Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward and backward. The controlling factor in. Why Don't Catalysts Affect Equilibrium.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3480898 Why Don't Catalysts Affect Equilibrium If the conditions of a. Hence, although the system will reach dynamic. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). Catalysts do not affect the position of equilibrium, they accelerate the respective (. Why Don't Catalysts Affect Equilibrium.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.21C Understand Why a Catalyst Does Not Affect Why Don't Catalysts Affect Equilibrium Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward and backward. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). If the conditions of a. Though this increase in reaction rate may cause a system to reach equilibrium more. Why Don't Catalysts Affect Equilibrium.
From byjus.com
Why do catalyst not affect equilibrium? Why Don't Catalysts Affect Equilibrium In such a reaction a catalyst speeds up both the forward and the backward reactions. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. Though this increase in reaction rate may cause a system to reach equilibrium more quickly (by speeding up the forward and. Catalysts have no effect on the equilibrium constant and. Why Don't Catalysts Affect Equilibrium.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free Why Don't Catalysts Affect Equilibrium The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward and backward. If the conditions of a. Though this increase in reaction rate may cause a system to reach equilibrium more. Why Don't Catalysts Affect Equilibrium.
From www.slideserve.com
PPT Factors that Affect Equilibrium PowerPoint Presentation, free Why Don't Catalysts Affect Equilibrium Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward and backward. Hence, although the system will reach dynamic. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and. Why Don't Catalysts Affect Equilibrium.
From slideplayer.com
Equilibrium Chapter ppt download Why Don't Catalysts Affect Equilibrium Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward and backward. If the conditions of a. Hence, although the system will reach dynamic. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium. Why Don't Catalysts Affect Equilibrium.
From hxeaspeqb.blob.core.windows.net
Why Do Catalysts Not Affect The Position Of Equilibrium at Erica Murphy Why Don't Catalysts Affect Equilibrium Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. In such a reaction a catalyst speeds up both the forward and the backward reactions. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). A catalyst speeds up both the forward and the reverse. Why Don't Catalysts Affect Equilibrium.
From slideplayer.com
Chapter 14 Chemical Equilibrium ppt download Why Don't Catalysts Affect Equilibrium Though this increase in reaction rate may cause a system to reach equilibrium more quickly (by speeding up the forward and. If the conditions of a. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. In such a reaction a catalyst speeds up both the forward and the backward reactions. A catalyst speeds up both. Why Don't Catalysts Affect Equilibrium.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free Why Don't Catalysts Affect Equilibrium In such a reaction a catalyst speeds up both the forward and the backward reactions. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. The controlling factor in where the equilibrium lies is the difference in free energy between the. Why Don't Catalysts Affect Equilibrium.
From slideplayer.com
Chemical Equilibrium ppt download Why Don't Catalysts Affect Equilibrium Hence, although the system will reach dynamic. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. In such a reaction a catalyst speeds up both the forward and the backward reactions. If the conditions of a. Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward. Why Don't Catalysts Affect Equilibrium.
From www.slideserve.com
PPT Chapter 13 Chemical Equilibrium PowerPoint Presentation, free Why Don't Catalysts Affect Equilibrium The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). Hence, although the system will reach dynamic. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. If the conditions of a. In such a reaction a catalyst speeds. Why Don't Catalysts Affect Equilibrium.
From slideplayer.com
CHEMICAL EQUILIBRIUM ppt download Why Don't Catalysts Affect Equilibrium Hence, although the system will reach dynamic. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Though this increase in reaction rate may cause a system to reach equilibrium more quickly (by speeding up the forward and. Catalysts do not affect the position of equilibrium, they accelerate the respective. Why Don't Catalysts Affect Equilibrium.
From www.slideserve.com
PPT Rates of Reaction and Chemical Equilibrium PowerPoint Why Don't Catalysts Affect Equilibrium Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Though this increase in reaction rate may cause a system to reach equilibrium more quickly (by speeding up the forward and. Hence, although the system will reach dynamic. Catalysts do not. Why Don't Catalysts Affect Equilibrium.
From www.numerade.com
SOLVED6 Which explanation best describes why a catalyst does not Why Don't Catalysts Affect Equilibrium In such a reaction a catalyst speeds up both the forward and the backward reactions. Hence, although the system will reach dynamic. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Though this increase. Why Don't Catalysts Affect Equilibrium.
From slideplayer.com
Equilibrium Chapter ppt download Why Don't Catalysts Affect Equilibrium Hence, although the system will reach dynamic. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward. Why Don't Catalysts Affect Equilibrium.
From slideplayer.com
*Le Châtelier’s Principle and Equilibrium ppt download Why Don't Catalysts Affect Equilibrium Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward and backward. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). Hence, although the system will reach dynamic. Catalysts have no effect on the equilibrium constant and thus on the. Why Don't Catalysts Affect Equilibrium.
From www.slideserve.com
PPT Factors that Affect Equilibrium PowerPoint Presentation, free Why Don't Catalysts Affect Equilibrium Hence, although the system will reach dynamic. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. In such a reaction a catalyst speeds up both the forward and the backward reactions. Though this increase in reaction rate may cause a system to reach equilibrium more quickly (by speeding up the forward and. A catalyst. Why Don't Catalysts Affect Equilibrium.
From slideplayer.com
& Equilibrium ppt download Why Don't Catalysts Affect Equilibrium Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward and backward. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Though this increase in reaction rate may cause a system to reach equilibrium more quickly (by speeding up the forward and. If the conditions of a.. Why Don't Catalysts Affect Equilibrium.
From slideplayer.com
Chemical Equilibrium Chapter ppt download Why Don't Catalysts Affect Equilibrium Hence, although the system will reach dynamic. Though this increase in reaction rate may cause a system to reach equilibrium more quickly (by speeding up the forward and. In such a reaction a catalyst speeds up both the forward and the backward reactions. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change. Why Don't Catalysts Affect Equilibrium.
From slideplayer.com
Chemical Equilibrium Chapter ppt download Why Don't Catalysts Affect Equilibrium Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward and backward. Hence, although the system will reach dynamic. If the conditions of a. In such a reaction a catalyst speeds up both the forward and the backward reactions. The controlling factor in where the equilibrium lies is the difference in free. Why Don't Catalysts Affect Equilibrium.
From byjus.com
3. How does catalysts affect The equilibrium? Why Don't Catalysts Affect Equilibrium Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). In such a reaction a. Why Don't Catalysts Affect Equilibrium.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3480898 Why Don't Catalysts Affect Equilibrium Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. Catalysts do not affect the position of equilibrium, they accelerate the. Why Don't Catalysts Affect Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Why Don't Catalysts Affect Equilibrium Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. If the conditions of a. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Hence, although the system will reach. Why Don't Catalysts Affect Equilibrium.
From slideplayer.com
Factors That Affect Equilibrium ppt download Why Don't Catalysts Affect Equilibrium Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward and backward. Hence, although the system will reach dynamic. If the conditions of a. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). Though this increase in reaction rate may. Why Don't Catalysts Affect Equilibrium.
From www.chegg.com
Solved Understand how catalysts affect equilibrium Question Why Don't Catalysts Affect Equilibrium A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Hence, although the system will reach dynamic. In such a reaction a catalyst speeds up both the forward and the backward reactions. Catalysts do not affect the position of equilibrium, they accelerate the respective ( and possibly selected ) forward. Why Don't Catalysts Affect Equilibrium.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID2796960 Why Don't Catalysts Affect Equilibrium Hence, although the system will reach dynamic. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. In such a reaction a catalyst speeds up both the forward and the backward reactions. If the conditions of a. The controlling factor in. Why Don't Catalysts Affect Equilibrium.
From www.youtube.com
7.1/R2.2 State and Explain the Effect of a Catalyst on an Equilibrium Why Don't Catalysts Affect Equilibrium Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Hence, although the system will reach dynamic. If the conditions of a. Catalysts do not affect the position of equilibrium, they accelerate the respective (. Why Don't Catalysts Affect Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Why Don't Catalysts Affect Equilibrium If the conditions of a. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. In such a reaction a catalyst speeds up both the forward and the backward reactions. Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. A catalyst speeds up both the forward and the reverse reactions,. Why Don't Catalysts Affect Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Why Don't Catalysts Affect Equilibrium If the conditions of a. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. The controlling factor in where the equilibrium lies is the difference in free energy between the reactants and products (the δg). Though this increase in reaction rate may cause a system to reach equilibrium more. Why Don't Catalysts Affect Equilibrium.
From slideplayer.com
Chemical Equilibrium Chapter ppt download Why Don't Catalysts Affect Equilibrium A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Hence, although the system will reach dynamic. If the conditions of a. Though this increase in reaction rate may cause a system to reach equilibrium more. Why Don't Catalysts Affect Equilibrium.
From www.numerade.com
⏩SOLVEDDoes a catalyst affect the value of the equilibrium… Numerade Why Don't Catalysts Affect Equilibrium Catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible reaction. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Though this increase in reaction rate may cause a system to. Why Don't Catalysts Affect Equilibrium.