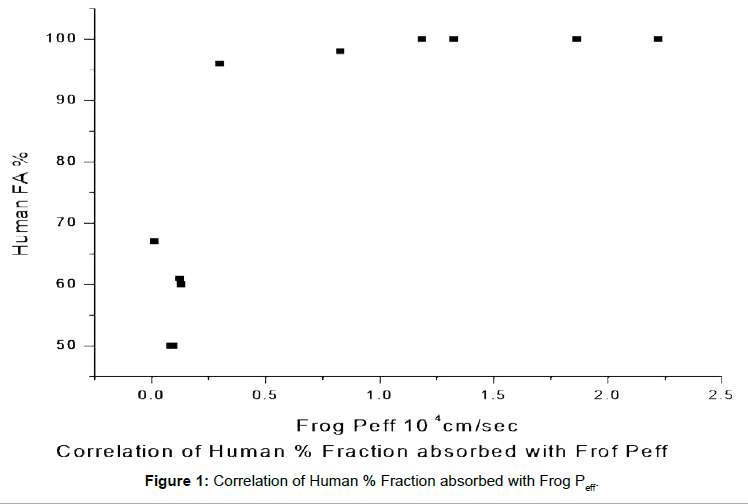
Animal Model Bioavailability . We attempted to establish animal models to evaluate the effects of drug degradation in the stomach on oral bioavailability. In addition, we assessed the utilization of. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. Animal models are considered as most important in vivo models in terms of.
from www.walshmedicalmedia.com
In addition, we assessed the utilization of. We attempted to establish animal models to evaluate the effects of drug degradation in the stomach on oral bioavailability. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. Animal models are considered as most important in vivo models in terms of.
Evaluation of Frog as an Animal Model to Study the Fraction of Or
Animal Model Bioavailability Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. Animal models are considered as most important in vivo models in terms of. We attempted to establish animal models to evaluate the effects of drug degradation in the stomach on oral bioavailability. In addition, we assessed the utilization of. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted.
From www.walshmedicalmedia.com
Evaluation of Frog as an Animal Model to Study the Fraction of Or Animal Model Bioavailability Animal models are considered as most important in vivo models in terms of. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. We attempted to establish animal models to evaluate the effects of drug degradation. Animal Model Bioavailability.
From www.mdpi.com
Agriculture Free FullText Phosphorus Bioavailability A Key Aspect Animal Model Bioavailability Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. We attempted to establish animal models to evaluate the effects of drug degradation in the stomach on oral bioavailability. In addition, we assessed the utilization of. The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have. Animal Model Bioavailability.
From www.researchgate.net
Selection of Different Animal Models Used for Investigating Aspects of Animal Model Bioavailability Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. In addition, we assessed the utilization of. The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. Due to the limited bioavailability, selection. Animal Model Bioavailability.
From www.academia.edu
(PDF) Bioavailability of zinc glycinate in comparison with zinc Animal Model Bioavailability In addition, we assessed the utilization of. Animal models are considered as most important in vivo models in terms of. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. Objectives in pharmaceutical drug development, preclinical tests. Animal Model Bioavailability.
From www.mdpi.com
Swine as the Animal Model for Testing New Formulations of Anti Animal Model Bioavailability The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. In addition, we assessed the utilization of. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. We attempted to establish animal models to evaluate the effects of drug degradation in the stomach on oral bioavailability. Due to the limited. Animal Model Bioavailability.
From www.academia.edu
(PDF) Effects of condensed organic matter on PCBs bioavailability in Animal Model Bioavailability We attempted to establish animal models to evaluate the effects of drug degradation in the stomach on oral bioavailability. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. In addition, we assessed the utilization of.. Animal Model Bioavailability.
From www.researchgate.net
(PDF) Swine as the Animal Model for Testing New Formulations of Anti Animal Model Bioavailability Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. In addition, we assessed. Animal Model Bioavailability.
From www.researchgate.net
(PDF) DETERMINATION OF BIOAVAILABILITY (BA) IN ANIMAL MODELS (MICE Animal Model Bioavailability Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. In addition, we assessed the utilization of. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Animal models play a crucial role in advancing biomedical research, especially in the field. Animal Model Bioavailability.
From www.mdpi.com
Animals Free FullText The Importance of Animal Models in Animal Model Bioavailability We attempted to establish animal models to evaluate the effects of drug degradation in the stomach on oral bioavailability. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Animal models play a crucial role. Animal Model Bioavailability.
From jpionline.org
Exploration of Sublingual Route for the Enhanced Animal Model Bioavailability Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. In addition, we assessed the utilization of. In pharmaceutical. Animal Model Bioavailability.
From www.mdpi.com
Medicina Free FullText Bioavailability of Different Vitamin D Oral Animal Model Bioavailability Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. In pharmaceutical drug development, preclinical tests in. Animal Model Bioavailability.
From www.mdpi.com
Swine as the Animal Model for Testing New Formulations of Anti Animal Model Bioavailability In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Animal models are considered as most important in vivo models in terms of. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. In addition, we assessed the utilization of. Due to the limited. Animal Model Bioavailability.
From www.mdpi.com
Swine as the Animal Model for Testing New Formulations of Anti Animal Model Bioavailability The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. Animal models are considered as most important in vivo models in terms of. Animal models play a crucial role in advancing biomedical research, especially in. Animal Model Bioavailability.
From www.mdpi.com
Swine as the Animal Model for Testing New Formulations of Anti Animal Model Bioavailability Animal models are considered as most important in vivo models in terms of. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Companies are moving therapeutic testing from plastic wells. Animal Model Bioavailability.
From www.mdpi.com
Swine as the Animal Model for Testing New Formulations of Anti Animal Model Bioavailability Animal models are considered as most important in vivo models in terms of. The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. Animal models play a crucial role in advancing biomedical research, especially in. Animal Model Bioavailability.
From www.semanticscholar.org
Figure 1 from Quantitative prediction of human oral bioavailability Animal Model Bioavailability We attempted to establish animal models to evaluate the effects of drug degradation in the stomach on oral bioavailability. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Due to the limited. Animal Model Bioavailability.
From www.researchgate.net
(PDF) Neuroprotective Effect of Turmeric Extract in Combination with Animal Model Bioavailability Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Animal models are considered as most. Animal Model Bioavailability.
From www.researchgate.net
Bioavailability total effect of the CAT based model for neutral Animal Model Bioavailability The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. In addition, we assessed the utilization of. We attempted to. Animal Model Bioavailability.
From curtiscoulter.com
Combining InVivo Animal Models with Physiologically Based Animal Model Bioavailability Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. In addition, we assessed the utilization of. The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. In pharmaceutical drug development, preclinical tests. Animal Model Bioavailability.
From pubs.acs.org
Arsenic Relative Bioavailability in Contaminated Soils Comparison of Animal Model Bioavailability Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. Animal models are considered as most important in vivo models in terms of. In addition, we assessed the utilization of. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. In pharmaceutical drug. Animal Model Bioavailability.
From pharmwarthegame.blogspot.com
Bioavailability Animal Model Bioavailability Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. The remarkable anatomical. Animal Model Bioavailability.
From encyclopedia.pub
Bioavailability of Thymol in Humans and Animals Encyclopedia MDPI Animal Model Bioavailability We attempted to establish animal models to evaluate the effects of drug degradation in the stomach on oral bioavailability. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Companies are. Animal Model Bioavailability.
From www.researchgate.net
(PDF) Bioavailability of Different Vitamin D Oral Supplements in Animal Model Bioavailability Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. In addition, we assessed the utilization of. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the. Animal Model Bioavailability.
From www.walshmedicalmedia.com
Evaluation of Frog as an Animal Model to Study the Fraction of Or Animal Model Bioavailability Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. We attempted to establish animal models to evaluate the effects of drug degradation in the stomach on oral bioavailability. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally.. Animal Model Bioavailability.
From www.mdpi.com
Medicina Free FullText Bioavailability of Different Vitamin D Oral Animal Model Bioavailability The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. In addition, we assessed the. Animal Model Bioavailability.
From www.researchgate.net
(PDF) Animal Models Used for Bioavailability and Bioequivalence Studies Animal Model Bioavailability In addition, we assessed the utilization of. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Animal models are considered as most important in vivo models in terms of. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. Objectives in pharmaceutical drug development,. Animal Model Bioavailability.
From www.frontiersin.org
Frontiers Prospects of animal models and their application in studies Animal Model Bioavailability Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. In addition, we assessed the utilization of. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. We attempted to establish animal models to evaluate the effects of drug degradation in the stomach on oral bioavailability. Objectives. Animal Model Bioavailability.
From www.researchgate.net
(PDF) Arsenic relative bioavailability in contaminated soils Animal Model Bioavailability In addition, we assessed the utilization of. Animal models are considered as most important in vivo models in terms of. Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is. Animal Model Bioavailability.
From www.researchgate.net
(PDF) Bioavailability of Different Vitamin D Oral Supplements in Animal Model Bioavailability The remarkable anatomical and physiological similarities between humans and animals, particularly mammals, have prompted. Animal models are considered as most important in vivo models in terms of. Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug. Animal Model Bioavailability.
From www.researchgate.net
Summary of the bioavailability model for nickel carcinogenicity Animal Model Bioavailability In addition, we assessed the utilization of. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. We attempted to establish animal models to evaluate the effects of drug degradation in the stomach on oral bioavailability. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new. Animal Model Bioavailability.
From jpionline.org
Exploration of Sublingual Route for the Enhanced Animal Model Bioavailability Companies are moving therapeutic testing from plastic wells into pooled in vivo screening, generating physiologically relevant. Animal models are considered as most important in vivo models in terms of. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential. Animal Model Bioavailability.
From tiracole.com
Factors affecting protein bioavailability Tira's Corner Animal Model Bioavailability Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. In addition, we. Animal Model Bioavailability.
From www.researchgate.net
Assessment of bioavailability and animal behavior upon SAMtreatment Animal Model Bioavailability In addition, we assessed the utilization of. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. Objectives in pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Animal models are considered as most important in vivo models in terms of. We attempted to. Animal Model Bioavailability.
From typeset.io
(Open Access) Animal models for evaluation of oral delivery of Animal Model Bioavailability In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. Animal models are considered as most important in vivo models in terms of. Objectives in pharmaceutical drug development, preclinical tests. Animal Model Bioavailability.
From www.researchgate.net
In vitro Caco2 cell model (A) and the determined bioavailability (B Animal Model Bioavailability Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. In pharmaceutical drug development, preclinical tests in animal models are essential to demonstrate whether the new drug is orally. Due to the limited bioavailability, selection of the correct animal model and experimental settings are 73 key elements when evaluating oral. Companies are moving. Animal Model Bioavailability.