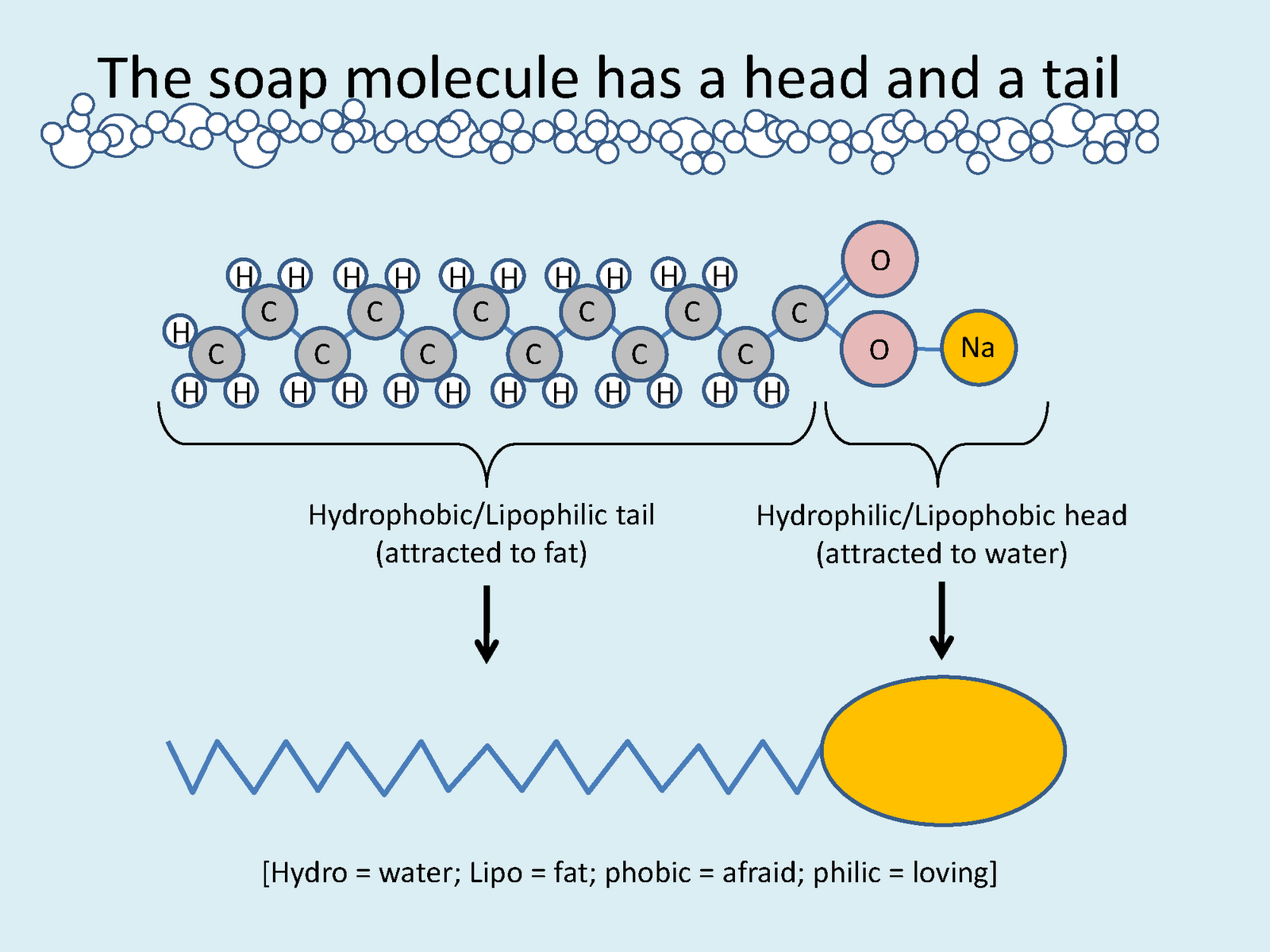
How To Make Soap Chemistry Experiment . Only small quantities of reagents are required, reducing the risks associated with the. Testing shower gels and soaps. This hydrolysis is called saponification, and the reaction has been known for centuries. Make lye soap from sodium hydroxide and olive oil via the. You will precipitate the soap by adding it to a. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. In this experiment, students use sodium hydroxide or sulfuric acid to make their own soap or detergent. Not only is it a process that uses science,. Soap can be made from the base hydrolysis of a fat or an oil. The objective of this laboratory is to make lye soap via the saponification reaction. Soap making has remained unchanged over the centuries. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules.
from www.emaze.com
You will precipitate the soap by adding it to a. Only small quantities of reagents are required, reducing the risks associated with the. In this experiment, students use sodium hydroxide or sulfuric acid to make their own soap or detergent. Testing shower gels and soaps. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. Soap can be made from the base hydrolysis of a fat or an oil. The objective of this laboratory is to make lye soap via the saponification reaction. Soap making has remained unchanged over the centuries. Not only is it a process that uses science,. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide.
Presentation Name on emaze
How To Make Soap Chemistry Experiment This hydrolysis is called saponification, and the reaction has been known for centuries. The objective of this laboratory is to make lye soap via the saponification reaction. Only small quantities of reagents are required, reducing the risks associated with the. Soap making has remained unchanged over the centuries. Testing shower gels and soaps. Make lye soap from sodium hydroxide and olive oil via the. You will precipitate the soap by adding it to a. This hydrolysis is called saponification, and the reaction has been known for centuries. Soap can be made from the base hydrolysis of a fat or an oil. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. In this experiment, students use sodium hydroxide or sulfuric acid to make their own soap or detergent. Not only is it a process that uses science,. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide.
From preparecenter.org
How Soap Works Science Experiment PrepareCenter How To Make Soap Chemistry Experiment Not only is it a process that uses science,. Make lye soap from sodium hydroxide and olive oil via the. Only small quantities of reagents are required, reducing the risks associated with the. In this experiment, students use sodium hydroxide or sulfuric acid to make their own soap or detergent. You will precipitate the soap by adding it to a.. How To Make Soap Chemistry Experiment.
From www.youtube.com
Chemistry 101 How does soap work? YouTube How To Make Soap Chemistry Experiment Make lye soap from sodium hydroxide and olive oil via the. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. Soap can be made from. How To Make Soap Chemistry Experiment.
From homeschoolden.com
Chemistry Experiments for Kids (Grade 2) Mixtures, Chromatography How To Make Soap Chemistry Experiment Not only is it a process that uses science,. You will precipitate the soap by adding it to a. Make lye soap from sodium hydroxide and olive oil via the. In this experiment, students use sodium hydroxide or sulfuric acid to make their own soap or detergent. Soap can be made from the base hydrolysis of a fat or an. How To Make Soap Chemistry Experiment.
From www.pinterest.com
Soap Science Fair Project Science Fair Pinterest Mondays, Soaps How To Make Soap Chemistry Experiment You will precipitate the soap by adding it to a. Soap can be made from the base hydrolysis of a fat or an oil. Soap making has remained unchanged over the centuries. Testing shower gels and soaps. Not only is it a process that uses science,. In this experiment, students use sodium hydroxide or sulfuric acid to make their own. How To Make Soap Chemistry Experiment.
From ar.inspiredpencil.com
Preparation Of Soap In Chemistry Project How To Make Soap Chemistry Experiment The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. Soap making has remained unchanged over the centuries. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. This hydrolysis is called saponification, and the reaction. How To Make Soap Chemistry Experiment.
From www.numerade.com
SOLVED Preparation of Soap experiment chemistry 1. Why is the How To Make Soap Chemistry Experiment In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. You will precipitate the soap by adding it to a. Only small quantities of reagents are required, reducing the risks associated with the. The science behind soap making is in the structure of the fats, the properties of the lye, and. How To Make Soap Chemistry Experiment.
From learnphysics-dhruv.blogspot.com
Physics Learn To prepare Soap by cold process. Science practical GSEB How To Make Soap Chemistry Experiment Only small quantities of reagents are required, reducing the risks associated with the. Soap making has remained unchanged over the centuries. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. Make lye soap from sodium hydroxide and olive oil via the. Soap can be. How To Make Soap Chemistry Experiment.
From www.slideshare.net
How to make soap Experiment for Chemistry lessons How To Make Soap Chemistry Experiment This hydrolysis is called saponification, and the reaction has been known for centuries. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. Only small quantities of reagents are required, reducing the risks associated with the. Testing shower gels and soaps. Make lye soap from. How To Make Soap Chemistry Experiment.
From www.youtube.com
Preparation of soap from fat Chemistry YouTube How To Make Soap Chemistry Experiment Only small quantities of reagents are required, reducing the risks associated with the. Testing shower gels and soaps. You will precipitate the soap by adding it to a. The objective of this laboratory is to make lye soap via the saponification reaction. This hydrolysis is called saponification, and the reaction has been known for centuries. Make lye soap from sodium. How To Make Soap Chemistry Experiment.
From www.chemedx.org
Soap Making Chemical Education Xchange How To Make Soap Chemistry Experiment Testing shower gels and soaps. Soap can be made from the base hydrolysis of a fat or an oil. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. You will precipitate the soap by adding it to a. Soap making has remained unchanged over the centuries. This hydrolysis is called. How To Make Soap Chemistry Experiment.
From myprojectideas.com
Chemistry Experiments Archives My Project Ideas How To Make Soap Chemistry Experiment Make lye soap from sodium hydroxide and olive oil via the. This hydrolysis is called saponification, and the reaction has been known for centuries. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. The science behind soap making is in the structure of the fats, the properties of the lye,. How To Make Soap Chemistry Experiment.
From labmuffin.com
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab How To Make Soap Chemistry Experiment Testing shower gels and soaps. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. You will precipitate the soap by adding it to a. Soap can be made from the base hydrolysis of a fat or an oil. In this experiment, students use sodium hydroxide or sulfuric acid to make. How To Make Soap Chemistry Experiment.
From in.pinterest.com
Hand washing with soap vector illustration. Educational explanation How To Make Soap Chemistry Experiment Soap making has remained unchanged over the centuries. This hydrolysis is called saponification, and the reaction has been known for centuries. Not only is it a process that uses science,. Only small quantities of reagents are required, reducing the risks associated with the. The science behind soap making is in the structure of the fats, the properties of the lye,. How To Make Soap Chemistry Experiment.
From www.scribd.com
The Chemical Reaction of Soap Making Chemistry Physical Sciences How To Make Soap Chemistry Experiment The objective of this laboratory is to make lye soap via the saponification reaction. Only small quantities of reagents are required, reducing the risks associated with the. Soap making has remained unchanged over the centuries. You will precipitate the soap by adding it to a. Soap can be made from the base hydrolysis of a fat or an oil. The. How To Make Soap Chemistry Experiment.
From www.slideshare.net
Chemistry of soaps How To Make Soap Chemistry Experiment This hydrolysis is called saponification, and the reaction has been known for centuries. In this experiment, students use sodium hydroxide or sulfuric acid to make their own soap or detergent. Make lye soap from sodium hydroxide and olive oil via the. Not only is it a process that uses science,. The science behind soap making is in the structure of. How To Make Soap Chemistry Experiment.
From www.youtube.com
Effect of Sodium Carbonate on Foaming Capacity of Soap Chemistry How To Make Soap Chemistry Experiment Testing shower gels and soaps. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. Not only is it a process that uses science,. Soap can be made from the base hydrolysis of a fat or an oil. You will precipitate the soap by adding. How To Make Soap Chemistry Experiment.
From www.youtube.com
Saponification Making Soap YouTube How To Make Soap Chemistry Experiment Make lye soap from sodium hydroxide and olive oil via the. In this experiment, students use sodium hydroxide or sulfuric acid to make their own soap or detergent. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. The objective of this laboratory is to make lye soap via the saponification. How To Make Soap Chemistry Experiment.
From www.youtube.com
Chemistry project file on "foaming capacity of soap" YouTube How To Make Soap Chemistry Experiment The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. Testing shower gels and soaps. In this experiment, students use sodium hydroxide or sulfuric acid to make their own soap or detergent. This hydrolysis is called saponification, and the reaction has been known for centuries.. How To Make Soap Chemistry Experiment.
From www.pinterest.com
Soap Experiments for Kids Science fair, Science fair projects How To Make Soap Chemistry Experiment Testing shower gels and soaps. Not only is it a process that uses science,. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. Soap making has remained unchanged over the centuries. Only small quantities of reagents are required, reducing the risks associated with the. In this experiment, students use sodium. How To Make Soap Chemistry Experiment.
From www.slideshare.net
How to make soap Experiment for Chemistry lessons How To Make Soap Chemistry Experiment In this experiment, students use sodium hydroxide or sulfuric acid to make their own soap or detergent. The objective of this laboratory is to make lye soap via the saponification reaction. Soap can be made from the base hydrolysis of a fat or an oil. Not only is it a process that uses science,. The science behind soap making is. How To Make Soap Chemistry Experiment.
From www.instructables.com
Making Soap With Chemistry 6 Steps Instructables How To Make Soap Chemistry Experiment Only small quantities of reagents are required, reducing the risks associated with the. The objective of this laboratory is to make lye soap via the saponification reaction. You will precipitate the soap by adding it to a. In this experiment, students use sodium hydroxide or sulfuric acid to make their own soap or detergent. The science behind soap making is. How To Make Soap Chemistry Experiment.
From busy.org
Saponification 1/2 How To Make Soap Chemistry Experiment You will precipitate the soap by adding it to a. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. The objective of this laboratory is to make lye soap via the saponification reaction. Not only is it a process that uses science,. In this. How To Make Soap Chemistry Experiment.
From joitjxuuu.blob.core.windows.net
Making Soap In Chemistry Class at Willis Jones blog How To Make Soap Chemistry Experiment Only small quantities of reagents are required, reducing the risks associated with the. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. The objective of this laboratory is to make lye soap via the saponification reaction. Soap can be made from the base hydrolysis of a fat or an oil.. How To Make Soap Chemistry Experiment.
From lilis-chemistryblog.weebly.com
Soap Making Chemistry Blog How To Make Soap Chemistry Experiment This hydrolysis is called saponification, and the reaction has been known for centuries. You will precipitate the soap by adding it to a. Soap making has remained unchanged over the centuries. Not only is it a process that uses science,. The objective of this laboratory is to make lye soap via the saponification reaction. In this experiment, you will make. How To Make Soap Chemistry Experiment.
From adabofgluewilldo.com
Ivory Soap Microwave Experiment How To Make Soap Chemistry Experiment In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. In this experiment, students use sodium hydroxide or sulfuric acid to make their own soap or detergent. Not only is it a process that uses science,. This hydrolysis is called saponification, and the reaction has been known for centuries. The objective. How To Make Soap Chemistry Experiment.
From www.youtube.com
Hydrolysis & How It Is Used To Make Soaps Organic Chemistry How To Make Soap Chemistry Experiment The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. In this experiment, students use sodium hydroxide or sulfuric acid to make their own soap or detergent. Not only is it a process that uses science,. Soap can be made from the base hydrolysis of. How To Make Soap Chemistry Experiment.
From www.pinterest.com
Top 10 Best Chemistry Project of Science Exhibition Chemistry How To Make Soap Chemistry Experiment Not only is it a process that uses science,. Make lye soap from sodium hydroxide and olive oil via the. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. The objective of this laboratory is to make lye soap via the saponification reaction. Testing shower gels and soaps. Only small. How To Make Soap Chemistry Experiment.
From scienceexperimentsanimated.blogspot.com
Science fair Experiments Soaps and detergents for std 8 to 10 Science How To Make Soap Chemistry Experiment The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. You will precipitate the soap by adding it to a. The objective of this laboratory is to make lye soap via the saponification reaction. In this experiment, you will make soap from a fat or. How To Make Soap Chemistry Experiment.
From www.simplykinder.com
Growing Ivory Soap Science Experiment Simply Kinder How To Make Soap Chemistry Experiment Testing shower gels and soaps. The objective of this laboratory is to make lye soap via the saponification reaction. Soap can be made from the base hydrolysis of a fat or an oil. Not only is it a process that uses science,. Soap making has remained unchanged over the centuries. Make lye soap from sodium hydroxide and olive oil via. How To Make Soap Chemistry Experiment.
From www.slideshare.net
Foaming Capacity of Soap How To Make Soap Chemistry Experiment You will precipitate the soap by adding it to a. Testing shower gels and soaps. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. The objective of this laboratory is to make lye soap via the saponification reaction. Soap making has remained unchanged over the centuries. Only small quantities of. How To Make Soap Chemistry Experiment.
From ar.inspiredpencil.com
Preparation Of Soap In Chemistry Project How To Make Soap Chemistry Experiment Not only is it a process that uses science,. Testing shower gels and soaps. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. Only small quantities of reagents are required, reducing the risks associated with the. Soap can be made from the base hydrolysis of a fat or an oil.. How To Make Soap Chemistry Experiment.
From www.emaze.com
Presentation Name on emaze How To Make Soap Chemistry Experiment You will precipitate the soap by adding it to a. The objective of this laboratory is to make lye soap via the saponification reaction. In this experiment, students use sodium hydroxide or sulfuric acid to make their own soap or detergent. Make lye soap from sodium hydroxide and olive oil via the. The science behind soap making is in the. How To Make Soap Chemistry Experiment.
From cbse.myindialist.com
Chemistry X Carbon and its Compounds SOAPS AND DETERGENTS CBSE How To Make Soap Chemistry Experiment Not only is it a process that uses science,. Soap can be made from the base hydrolysis of a fat or an oil. Soap making has remained unchanged over the centuries. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. This hydrolysis is called saponification, and the reaction has been. How To Make Soap Chemistry Experiment.
From crusadernews.com
Chemistry students design, create soaps Crusader News How To Make Soap Chemistry Experiment Only small quantities of reagents are required, reducing the risks associated with the. The objective of this laboratory is to make lye soap via the saponification reaction. Not only is it a process that uses science,. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. You will precipitate the soap. How To Make Soap Chemistry Experiment.
From www.pinterest.com
Making Soap With Chemistry!! Soap making, Soap, Steam projects How To Make Soap Chemistry Experiment Testing shower gels and soaps. Make lye soap from sodium hydroxide and olive oil via the. Soap can be made from the base hydrolysis of a fat or an oil. Only small quantities of reagents are required, reducing the risks associated with the. Not only is it a process that uses science,. This hydrolysis is called saponification, and the reaction. How To Make Soap Chemistry Experiment.