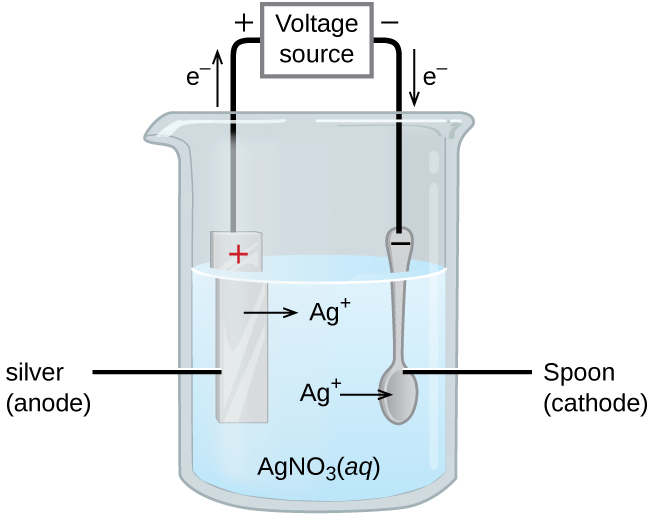
When Electrodes Are Immersed In Water And Electricity Passed . An electrical current is passed through water, splitting the water into hydrogen and oxygen gases. What happens when electrodes are immersed in water and current is passed through it? If electrodes are immersed in water, and a current is passed, bubbles of oxygen and hydrogen are produced. Oxygen bubbles forms on the. When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. When electrodes are immersed in water and current is passed through. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as. During the process of water electrolysis,. The correct option is a hydrogen. Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. If electrodes connected to battery terminals are placed in liquid.
from philschatz.com
If electrodes connected to battery terminals are placed in liquid. What happens when electrodes are immersed in water and current is passed through it? Oxygen bubbles forms on the. The correct option is a hydrogen. When electrodes are immersed in water and current is passed through. During the process of water electrolysis,. When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. An electrical current is passed through water, splitting the water into hydrogen and oxygen gases. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases.
Electrolysis · Chemistry
When Electrodes Are Immersed In Water And Electricity Passed During the process of water electrolysis,. When electrodes are immersed in water and current is passed through. The correct option is a hydrogen. During the process of water electrolysis,. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. If electrodes connected to battery terminals are placed in liquid. What happens when electrodes are immersed in water and current is passed through it? Oxygen bubbles forms on the. An electrical current is passed through water, splitting the water into hydrogen and oxygen gases. When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as. If electrodes are immersed in water, and a current is passed, bubbles of oxygen and hydrogen are produced. Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases.
From ourfuture.energy
Light from salt OurFuture.Energy When Electrodes Are Immersed In Water And Electricity Passed When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. When electrodes are immersed in water and current is passed through. If electrodes are immersed in water, and a current is passed, bubbles of. When Electrodes Are Immersed In Water And Electricity Passed.
From exotbpcqw.blob.core.windows.net
Does Electrolysis Work With Salt Water at Jean Woods blog When Electrodes Are Immersed In Water And Electricity Passed Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. What happens when electrodes are immersed in water and current is passed through it? The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as. The correct option is a hydrogen. When electrodes. When Electrodes Are Immersed In Water And Electricity Passed.
From chemistryfromscratch.org
V2.8 When Electrodes Are Immersed In Water And Electricity Passed When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. An electrical current is passed through water, splitting the water into hydrogen and oxygen gases. Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. If electrodes are immersed in water, and a current is passed,. When Electrodes Are Immersed In Water And Electricity Passed.
From www.electricautomationnetwork.com
5TG8223 SIEMENS Immersion electrode, pure water Electric A.. When Electrodes Are Immersed In Water And Electricity Passed The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as. If electrodes are immersed in water, and a current is passed, bubbles of oxygen and hydrogen are produced. What happens when electrodes are immersed in water and current is passed through it? Oxygen bubbles forms on the.. When Electrodes Are Immersed In Water And Electricity Passed.
From mantavya.com
What Is Electroplating & How does it work 2021 Guide Mantavya When Electrodes Are Immersed In Water And Electricity Passed If electrodes connected to battery terminals are placed in liquid. When electrodes are immersed in water and current is passed through. When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. What happens when electrodes are immersed in water and current is passed through it? The correct option is a hydrogen.. When Electrodes Are Immersed In Water And Electricity Passed.
From brainly.in
What happens when electric current is passed through acidulated water When Electrodes Are Immersed In Water And Electricity Passed Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. An electrical current is passed through water, splitting the water into hydrogen and oxygen gases. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. During the process of water electrolysis,. The correct option is a hydrogen. The. When Electrodes Are Immersed In Water And Electricity Passed.
From pineresearch.com
ThreeElectrode Setups Pine Research Instrumentation Store When Electrodes Are Immersed In Water And Electricity Passed If electrodes are immersed in water, and a current is passed, bubbles of oxygen and hydrogen are produced. During the process of water electrolysis,. If electrodes connected to battery terminals are placed in liquid. An electrical current is passed through water, splitting the water into hydrogen and oxygen gases. Oxygen bubbles forms on the. Electrolysis of water is the process. When Electrodes Are Immersed In Water And Electricity Passed.
From fixlibrarybaladinas3c.z4.web.core.windows.net
What Happens At The Cathode In Electrolysis When Electrodes Are Immersed In Water And Electricity Passed Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. When electrodes are immersed in water and current is passed through. The correct option is a hydrogen. An electrical current is passed through water, splitting the water into. When Electrodes Are Immersed In Water And Electricity Passed.
From www.windsorenergy.co.nz
Electric & Electrode Boilers Windsor Energy When Electrodes Are Immersed In Water And Electricity Passed The correct option is a hydrogen. If electrodes are immersed in water, and a current is passed, bubbles of oxygen and hydrogen are produced. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as. What happens when electrodes are immersed in water and current is passed through. When Electrodes Are Immersed In Water And Electricity Passed.
From brainly.in
When electrodes are immersed in water and electricity is passed, the When Electrodes Are Immersed In Water And Electricity Passed The correct option is a hydrogen. Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. If electrodes are immersed in water, and a current is passed, bubbles of oxygen and hydrogen are produced. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. Oxygen bubbles forms on. When Electrodes Are Immersed In Water And Electricity Passed.
From saylordotorg.github.io
Electrochemistry When Electrodes Are Immersed In Water And Electricity Passed When electrodes are immersed in water and current is passed through. Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. The correct option is a hydrogen. An electrical current is passed through water, splitting the water into hydrogen and oxygen gases. Oxygen bubbles forms on the. What happens when electrodes are immersed in water. When Electrodes Are Immersed In Water And Electricity Passed.
From askfilo.com
that if electrodes were immersed in water, and a current was passed. bubb.. When Electrodes Are Immersed In Water And Electricity Passed If electrodes are immersed in water, and a current is passed, bubbles of oxygen and hydrogen are produced. Oxygen bubbles forms on the. If electrodes connected to battery terminals are placed in liquid. When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. During the process of water electrolysis,. An electrical. When Electrodes Are Immersed In Water And Electricity Passed.
From courses.lumenlearning.com
Electrolytes General Chemistry When Electrodes Are Immersed In Water And Electricity Passed What happens when electrodes are immersed in water and current is passed through it? If electrodes are immersed in water, and a current is passed, bubbles of oxygen and hydrogen are produced. The correct option is a hydrogen. If electrodes connected to battery terminals are placed in liquid. Electrolysis of water is the process of using electricity to decompose water. When Electrodes Are Immersed In Water And Electricity Passed.
From saylordotorg.github.io
Standard Potentials When Electrodes Are Immersed In Water And Electricity Passed When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. An electrical current is passed through water, splitting the. When Electrodes Are Immersed In Water And Electricity Passed.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at When Electrodes Are Immersed In Water And Electricity Passed What happens when electrodes are immersed in water and current is passed through it? If electrodes connected to battery terminals are placed in liquid. During the process of water electrolysis,. When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. The electrolytic cell consists of a pair of platinum electrodes immersed. When Electrodes Are Immersed In Water And Electricity Passed.
From watertechnologyreport.wordpress.com
Electrode Boilers Water Technology Report When Electrodes Are Immersed In Water And Electricity Passed An electrical current is passed through water, splitting the water into hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as. If electrodes connected to battery terminals are placed in liquid. If electrodes are immersed in water, and a current is passed, bubbles. When Electrodes Are Immersed In Water And Electricity Passed.
From www.toppr.com
Acidified water was electrolysed using an inert electrode and volume of When Electrodes Are Immersed In Water And Electricity Passed Oxygen bubbles forms on the. What happens when electrodes are immersed in water and current is passed through it? Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. The correct option is a hydrogen. Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. During the process. When Electrodes Are Immersed In Water And Electricity Passed.
From philschatz.com
Electrolytes · Chemistry When Electrodes Are Immersed In Water And Electricity Passed Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. Oxygen bubbles forms on the. When electrodes are immersed in water and current is passed through. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as. What happens when electrodes are immersed. When Electrodes Are Immersed In Water And Electricity Passed.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts When Electrodes Are Immersed In Water And Electricity Passed The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as. The correct option is a hydrogen. If electrodes are immersed in water, and a current is passed, bubbles of oxygen and hydrogen are produced. Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o. When Electrodes Are Immersed In Water And Electricity Passed.
From www.mdpi.com
Processes Free FullText CFD Modeling and Experimental Validation When Electrodes Are Immersed In Water And Electricity Passed When electrodes are immersed in water and current is passed through. During the process of water electrolysis,. The correct option is a hydrogen. An electrical current is passed through water, splitting the water into hydrogen and oxygen gases. If electrodes are immersed in water, and a current is passed, bubbles of oxygen and hydrogen are produced. Ions move to opposite. When Electrodes Are Immersed In Water And Electricity Passed.
From www.toppr.com
Three faraday of electricity is passed through three electrolytic cells When Electrodes Are Immersed In Water And Electricity Passed When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. When electrodes are immersed in water and current is passed through. During the process of water electrolysis,. If electrodes connected to battery terminals are placed in liquid. Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2). When Electrodes Are Immersed In Water And Electricity Passed.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at When Electrodes Are Immersed In Water And Electricity Passed When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. What happens when electrodes are immersed in water and current is passed through it? The correct option is a hydrogen. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. An electrical current is. When Electrodes Are Immersed In Water And Electricity Passed.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts When Electrodes Are Immersed In Water And Electricity Passed Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. During the process of water electrolysis,. An electrical current is passed through water, splitting the water into hydrogen and oxygen gases. What happens when electrodes are immersed in water and current is passed through it? The electrolytic cell consists of a pair of. When Electrodes Are Immersed In Water And Electricity Passed.
From saylordotorg.github.io
Describing Electrochemical Cells When Electrodes Are Immersed In Water And Electricity Passed Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. If electrodes are immersed in water, and a current is passed, bubbles of oxygen and hydrogen are produced. When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. The electrolytic cell consists of a pair of. When Electrodes Are Immersed In Water And Electricity Passed.
From www.teachoo.com
What are Different Chemical Effects of Electric Current? Teachoo When Electrodes Are Immersed In Water And Electricity Passed When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. Oxygen bubbles forms on the. If electrodes connected to battery terminals are placed in liquid. Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. Electrolysis of water is the process of using electricity to decompose. When Electrodes Are Immersed In Water And Electricity Passed.
From gadgillab.berkeley.edu
Capacitative Deionization Gadgil Lab for Energy & Water Research UC When Electrodes Are Immersed In Water And Electricity Passed During the process of water electrolysis,. An electrical current is passed through water, splitting the water into hydrogen and oxygen gases. When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. When electrodes are immersed in water and current is passed through. If electrodes connected to battery terminals are placed in. When Electrodes Are Immersed In Water And Electricity Passed.
From www.chegg.com
Solved a) An external direct current power supply is When Electrodes Are Immersed In Water And Electricity Passed The correct option is a hydrogen. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as. Oxygen bubbles forms on the. If electrodes connected to battery terminals are placed in liquid. When electrodes are immersed in water and current is passed through. Electrolysis of water is the. When Electrodes Are Immersed In Water And Electricity Passed.
From www.youtube.com
When electrodes are immersed in water and electricity is passed, the When Electrodes Are Immersed In Water And Electricity Passed When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as. The. When Electrodes Are Immersed In Water And Electricity Passed.
From saylordotorg.github.io
Electrochemistry When Electrodes Are Immersed In Water And Electricity Passed The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as. The correct option is a hydrogen. When electrodes are immersed in water and current is passed through. Oxygen bubbles forms on the. If electrodes are immersed in water, and a current is passed, bubbles of oxygen and. When Electrodes Are Immersed In Water And Electricity Passed.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk When Electrodes Are Immersed In Water And Electricity Passed What happens when electrodes are immersed in water and current is passed through it? The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as. Oxygen bubbles forms on the. The correct option is a hydrogen. If electrodes connected to battery terminals are placed in liquid. If electrodes. When Electrodes Are Immersed In Water And Electricity Passed.
From fixlibrarygedwaaldebx.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram When Electrodes Are Immersed In Water And Electricity Passed Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. The correct option is a hydrogen. If electrodes connected. When Electrodes Are Immersed In Water And Electricity Passed.
From www.reddit.com
If you put a pair of electrodes in a pool of water, will the When Electrodes Are Immersed In Water And Electricity Passed If electrodes connected to battery terminals are placed in liquid. What happens when electrodes are immersed in water and current is passed through it? An electrical current is passed through water, splitting the water into hydrogen and oxygen gases. During the process of water electrolysis,. The correct option is a hydrogen. Ions move to opposite electrodes, releasing pure hydrogen (h. When Electrodes Are Immersed In Water And Electricity Passed.
From itechguide.pages.dev
Does Ice Conduct Electricity itechguide When Electrodes Are Immersed In Water And Electricity Passed When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal is actually hydrogen. An electrical current is passed through water, splitting the water into hydrogen and oxygen gases. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. If electrodes are immersed in water, and a current. When Electrodes Are Immersed In Water And Electricity Passed.
From www.teachoo.com
What are Different Chemical Effects of Electric Current? Teachoo When Electrodes Are Immersed In Water And Electricity Passed The correct option is a hydrogen. What happens when electrodes are immersed in water and current is passed through it? Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. When electrodes are immersed in water and current is passed through. If electrodes are immersed in water, and a current is passed, bubbles. When Electrodes Are Immersed In Water And Electricity Passed.
From philschatz.com
Electrolysis · Chemistry When Electrodes Are Immersed In Water And Electricity Passed What happens when electrodes are immersed in water and current is passed through it? Ions move to opposite electrodes, releasing pure hydrogen (h 2) and oxygen (o 2) gases. Oxygen bubbles forms on the. If electrodes connected to battery terminals are placed in liquid. When electrodes are immersed in water and electricity passed, the bubbles formed on the negative terminal. When Electrodes Are Immersed In Water And Electricity Passed.