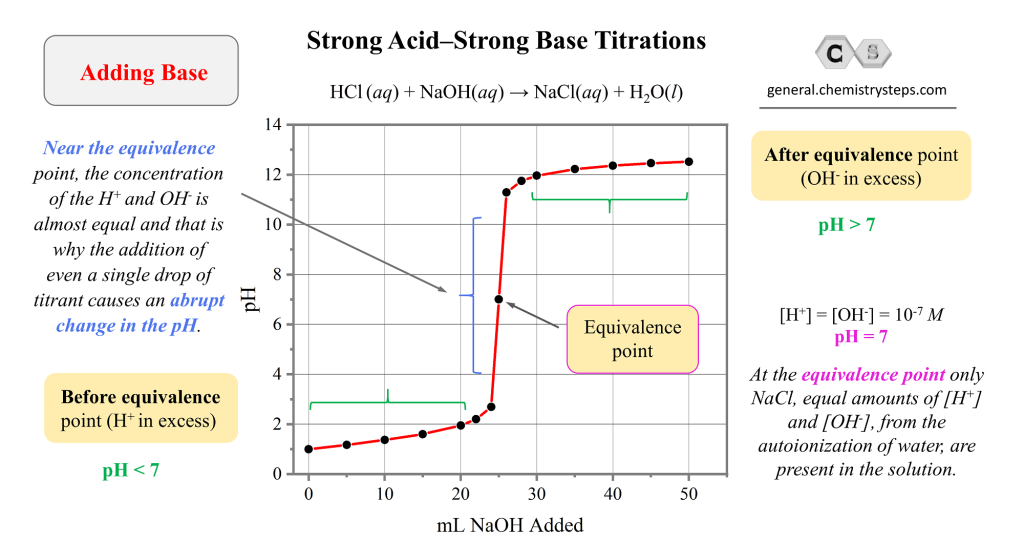
Strong Base Strong Acid Reaction . Explain the ph scale, and convert ph and concentration of. The acidity or basicity of an. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. Strong acid (hcl) plus strong base (naoh) giving water. The reaction of a strong acid with a strong base is essentially a quantitative reaction. Give the names and formulas of some strong acids and bases. This leads to complete neutralization, forming a. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). Strong acid with strong base:
from general.chemistrysteps.com
The reaction of a strong acid with a strong base is essentially a quantitative reaction. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). Strong acid (hcl) plus strong base (naoh) giving water. Strong acid with strong base: Give the names and formulas of some strong acids and bases. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. This leads to complete neutralization, forming a. Explain the ph scale, and convert ph and concentration of. The acidity or basicity of an. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt.
Strong AcidStrong Base Titrations Chemistry Steps
Strong Base Strong Acid Reaction The reaction of a strong acid with a strong base is essentially a quantitative reaction. Give the names and formulas of some strong acids and bases. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. The acidity or basicity of an. This leads to complete neutralization, forming a. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). Strong acid (hcl) plus strong base (naoh) giving water. Explain the ph scale, and convert ph and concentration of. Strong acid with strong base: The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. The reaction of a strong acid with a strong base is essentially a quantitative reaction.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Strong Base Strong Acid Reaction Explain the ph scale, and convert ph and concentration of. The acidity or basicity of an. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. Give the names and formulas of some strong acids and bases. Strong acid (hcl) plus strong base (naoh) giving water.. Strong Base Strong Acid Reaction.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Strong Base Strong Acid Reaction Strong acid with strong base: Strong acid (hcl) plus strong base (naoh) giving water. Give the names and formulas of some strong acids and bases. This leads to complete neutralization, forming a. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. The reaction of a. Strong Base Strong Acid Reaction.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation ID6155898 Strong Base Strong Acid Reaction This leads to complete neutralization, forming a. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Explain the ph scale, and convert ph and concentration of. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). Strong. Strong Base Strong Acid Reaction.
From www.teachoo.com
List of Strong Acids (More than 8 examples, with images) Teachoo Strong Base Strong Acid Reaction Explain the ph scale, and convert ph and concentration of. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. Strong acid (hcl) plus strong base (naoh) giving water. Strong acid with strong base: The reaction of a strong acid with a strong base is a. Strong Base Strong Acid Reaction.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID Strong Base Strong Acid Reaction This leads to complete neutralization, forming a. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). Give the names and formulas of some strong acids and bases. The acidity or basicity of an. The reaction of a strong acid with a strong base is a neutralization reaction,. Strong Base Strong Acid Reaction.
From www.masterorganicchemistry.com
How To Use a pKa Table Strong Base Strong Acid Reaction Explain the ph scale, and convert ph and concentration of. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). Strong acid with strong base: Nitric acid is. Strong Base Strong Acid Reaction.
From www.youtube.com
How To Memorize The Strong Acids and Strong Bases YouTube Strong Base Strong Acid Reaction Give the names and formulas of some strong acids and bases. Strong acid (hcl) plus strong base (naoh) giving water. Explain the ph scale, and convert ph and concentration of. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. The reaction of a strong acid. Strong Base Strong Acid Reaction.
From learningpectose.z13.web.core.windows.net
How To Do Acid Base Reactions Strong Base Strong Acid Reaction The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. Strong acid with strong base: The acidity or basicity of an. Give the names and formulas. Strong Base Strong Acid Reaction.
From www.alchem.ie
Equilibrium & Acid Base by Sarah Wegwerth Strong Base Strong Acid Reaction The acidity or basicity of an. The reaction of a strong acid with a strong base is essentially a quantitative reaction. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. Strong acid (hcl) plus strong base (naoh) giving water. Strong acid with strong base: The. Strong Base Strong Acid Reaction.
From www.youtube.com
Strong acidstrong base reactions Acids and bases AP Chemistry Strong Base Strong Acid Reaction The acidity or basicity of an. Strong acid (hcl) plus strong base (naoh) giving water. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Give. Strong Base Strong Acid Reaction.
From www.teachoo.com
Salts and it's Properties (with Examples) Acids, Bases and Salt Strong Base Strong Acid Reaction This leads to complete neutralization, forming a. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). The reaction of a strong acid with. Strong Base Strong Acid Reaction.
From www.slideserve.com
PPT Volumetric Analysis PowerPoint Presentation, free download ID Strong Base Strong Acid Reaction Strong acid (hcl) plus strong base (naoh) giving water. The reaction of a strong acid with a strong base is essentially a quantitative reaction. Explain the ph scale, and convert ph and concentration of. This leads to complete neutralization, forming a. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl. Strong Base Strong Acid Reaction.
From stock.adobe.com
Strong acid and strong base reaction. Strengths of acids and bases Strong Base Strong Acid Reaction Strong acid (hcl) plus strong base (naoh) giving water. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). This leads to complete neutralization, forming a. Explain the ph scale, and convert ph and concentration of. Nitric acid is hno3, and nitric acid reacts with water to form. Strong Base Strong Acid Reaction.
From www.slideserve.com
PPT CHAPTER 10 Reactions in Aqueous Solutions I Acids, Bases & Salts Strong Base Strong Acid Reaction Strong acid (hcl) plus strong base (naoh) giving water. Give the names and formulas of some strong acids and bases. The reaction of a strong acid with a strong base is essentially a quantitative reaction. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. Explain. Strong Base Strong Acid Reaction.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses Strong Base Strong Acid Reaction For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. The reaction of a strong acid with a strong base is a neutralization reaction,. Strong Base Strong Acid Reaction.
From www.khanacademy.org
Acidbase reactions (practice) Khan Academy Strong Base Strong Acid Reaction Strong acid (hcl) plus strong base (naoh) giving water. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). Explain the ph scale, and convert ph and concentration of. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus,. Strong Base Strong Acid Reaction.
From www.youtube.com
Weak acidstrong base reactions Acids and bases AP Chemistry Khan Strong Base Strong Acid Reaction The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Strong acid with strong base: For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). This leads to complete neutralization, forming a. Nitric acid is hno3, and nitric. Strong Base Strong Acid Reaction.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Strong Base Strong Acid Reaction Strong acid (hcl) plus strong base (naoh) giving water. Strong acid with strong base: The acidity or basicity of an. This leads to complete neutralization, forming a. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). Give the names and formulas of some strong acids and bases.. Strong Base Strong Acid Reaction.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6776086 Strong Base Strong Acid Reaction Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. Strong acid (hcl) plus strong base (naoh) giving water. This leads to complete neutralization, forming a. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl (. Strong Base Strong Acid Reaction.
From www.slideserve.com
PPT Ionization Constants of Acids and Bases Strengths of Acids and Strong Base Strong Acid Reaction Strong acid with strong base: The acidity or basicity of an. This leads to complete neutralization, forming a. Explain the ph scale, and convert ph and concentration of. The reaction of a strong acid with a strong base is essentially a quantitative reaction. Strong acid (hcl) plus strong base (naoh) giving water. For example, hcl ( aq ) + na. Strong Base Strong Acid Reaction.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID Strong Base Strong Acid Reaction For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). Explain the ph scale, and convert ph and concentration of. Strong acid (hcl) plus strong base (naoh) giving water. The acidity or basicity of an. This leads to complete neutralization, forming a. The reaction of a strong acid. Strong Base Strong Acid Reaction.
From www.slideserve.com
PPT Strong Acids & Bases PowerPoint Presentation, free download ID Strong Base Strong Acid Reaction The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. The acidity or basicity of an. Strong acid (hcl) plus strong base (naoh) giving water. This. Strong Base Strong Acid Reaction.
From www.slideserve.com
PPT Acids and bases, salts and solutions PowerPoint Presentation Strong Base Strong Acid Reaction The acidity or basicity of an. Strong acid (hcl) plus strong base (naoh) giving water. Explain the ph scale, and convert ph and concentration of. Give the names and formulas of some strong acids and bases. Strong acid with strong base: The reaction of a strong acid with a strong base is essentially a quantitative reaction. The reaction of a. Strong Base Strong Acid Reaction.
From stock.adobe.com
Strong acid and strong base reaction. Strengths of acids and bases Strong Base Strong Acid Reaction The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Give the names and formulas of some strong acids and bases. Strong acid (hcl) plus strong base (naoh) giving water. This leads to complete neutralization, forming a. Explain the ph scale, and convert ph and concentration of. For example, hcl. Strong Base Strong Acid Reaction.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo Strong Base Strong Acid Reaction Strong acid with strong base: Strong acid (hcl) plus strong base (naoh) giving water. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Give the names and formulas of some strong acids and bases. This leads to complete neutralization, forming a. Explain the ph scale, and convert ph and. Strong Base Strong Acid Reaction.
From www.masterorganicchemistry.com
Walkthrough of Acid Base Reactions (2) Basicity Master Organic Chemistry Strong Base Strong Acid Reaction Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. Strong acid (hcl) plus strong base (naoh) giving water. The acidity or basicity of an. The reaction of a strong acid with a strong base is essentially a quantitative reaction. This leads to complete neutralization, forming. Strong Base Strong Acid Reaction.
From www.shutterstock.com
9 Strong Acid And Weak Acid Dissociation Images, Stock Photos & Vectors Strong Base Strong Acid Reaction Explain the ph scale, and convert ph and concentration of. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. This leads to complete neutralization, forming a. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). Strong. Strong Base Strong Acid Reaction.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases Strong Base Strong Acid Reaction Strong acid (hcl) plus strong base (naoh) giving water. Explain the ph scale, and convert ph and concentration of. The acidity or basicity of an. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Give the names and formulas of some strong acids and bases. For example, hcl (. Strong Base Strong Acid Reaction.
From www.khanacademy.org
Acidbase reactions (practice) Khan Academy Strong Base Strong Acid Reaction Give the names and formulas of some strong acids and bases. This leads to complete neutralization, forming a. Strong acid with strong base: Strong acid (hcl) plus strong base (naoh) giving water. The acidity or basicity of an. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the. Strong Base Strong Acid Reaction.
From www.chegg.com
Solved REACTION OF ACIDS AND BASES 1. The reaction between a Strong Base Strong Acid Reaction Give the names and formulas of some strong acids and bases. Strong acid with strong base: Strong acid (hcl) plus strong base (naoh) giving water. The acidity or basicity of an. The reaction of a strong acid with a strong base is essentially a quantitative reaction. The reaction of a strong acid with a strong base is a neutralization reaction,. Strong Base Strong Acid Reaction.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID Strong Base Strong Acid Reaction Strong acid (hcl) plus strong base (naoh) giving water. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. This leads to complete neutralization, forming a. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). Strong acid. Strong Base Strong Acid Reaction.
From exoviutwi.blob.core.windows.net
What Happens When Strong Acid Reacts With Strong Base at Maude Higgins blog Strong Base Strong Acid Reaction The acidity or basicity of an. Explain the ph scale, and convert ph and concentration of. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). This leads to complete neutralization, forming a. Strong acid with strong base: Strong acid (hcl) plus strong base (naoh) giving water. Nitric. Strong Base Strong Acid Reaction.
From www.slideserve.com
PPT MODERN CHEMISTRY CHAPTER 14 ACIDS AND BASES PowerPoint Strong Base Strong Acid Reaction Strong acid with strong base: The reaction of a strong acid with a strong base is essentially a quantitative reaction. Give the names and formulas of some strong acids and bases. Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. Strong acid (hcl) plus strong. Strong Base Strong Acid Reaction.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID Strong Base Strong Acid Reaction The acidity or basicity of an. For example, hcl ( aq ) + na ( oh ) ( aq ) h 2 o + nacl ( aq ). Nitric acid is hno3, and nitric acid reacts with water to form hydronium, h3o plus, and nitrate, no3 minus, which is the conjugate base. Strong acid (hcl) plus strong base (naoh) giving. Strong Base Strong Acid Reaction.
From wisc.pb.unizin.org
Day 33 Acids and Bases Chemistry 109, Fall 2020 Strong Base Strong Acid Reaction This leads to complete neutralization, forming a. Strong acid with strong base: The acidity or basicity of an. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Give the names and formulas of some strong acids and bases. Explain the ph scale, and convert ph and concentration of. Nitric. Strong Base Strong Acid Reaction.