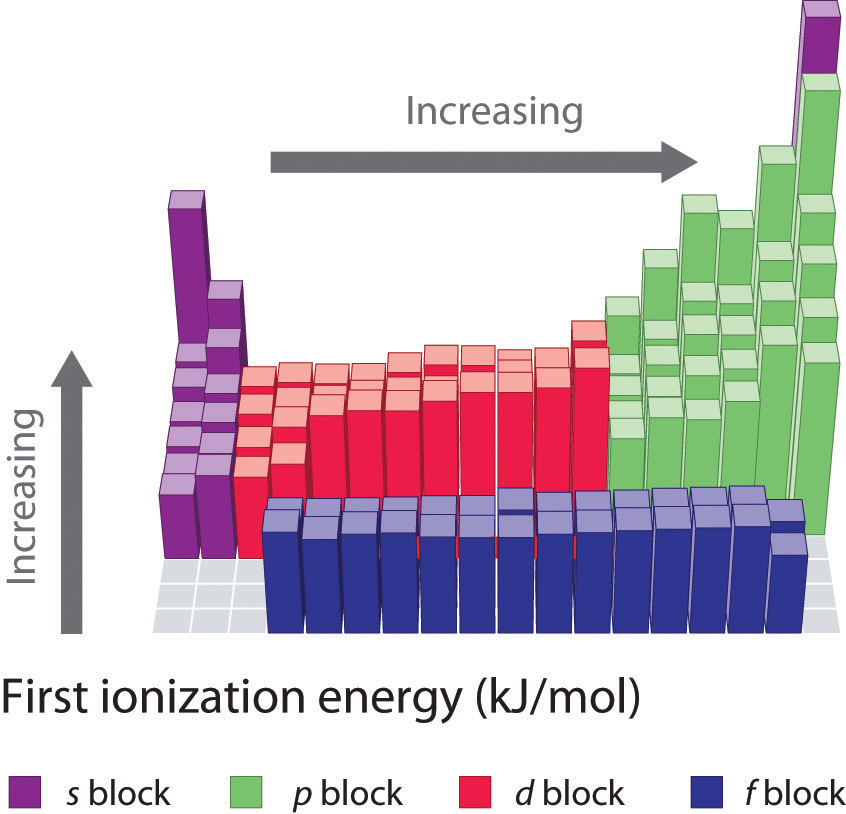
Chlorine Has A Higher Ionization Energy Than Fluorine . Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons are. No, fluorine has a higher ionization energy than chlorine. First ionization energy, second ionization energy as well as. Fluorine, which is higher up the group then chlorine, has a lower electron affinity. This makes the fluoride anion so. Li < b < be < c < n < o < f < ne. Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes more energy to remove the electron from fluorine than in chlorine. Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. 120 rows ionization energy chart of all the elements is given below. Fluorine is the most electronegative element in the periodic table,. This is because the electrons in the outermost.
from chem.libretexts.org
Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes more energy to remove the electron from fluorine than in chlorine. Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. Fluorine, which is higher up the group then chlorine, has a lower electron affinity. Fluorine is the most electronegative element in the periodic table,. First ionization energy, second ionization energy as well as. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. 120 rows ionization energy chart of all the elements is given below. This is because the electrons in the outermost. This makes the fluoride anion so. Li < b < be < c < n < o < f < ne.
7.4 Ionization Energy Chemistry LibreTexts
Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine, which is higher up the group then chlorine, has a lower electron affinity. This makes the fluoride anion so. Fluorine is the most electronegative element in the periodic table,. No, fluorine has a higher ionization energy than chlorine. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. This is because the electrons in the outermost. Li < b < be < c < n < o < f < ne. Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes more energy to remove the electron from fluorine than in chlorine. 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. Fluorine, which is higher up the group then chlorine, has a lower electron affinity. Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons are.
From sofiadonnell.z13.web.core.windows.net
Periodic Table Ionization Energy Chart Chlorine Has A Higher Ionization Energy Than Fluorine First ionization energy, second ionization energy as well as. No, fluorine has a higher ionization energy than chlorine. Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes more energy to remove the electron from fluorine than in chlorine. This is because the electrons in the outermost. 120 rows ionization energy chart of. Chlorine Has A Higher Ionization Energy Than Fluorine.
From ben-jolpbloghunter.blogspot.com
Why Does Fluorine Have a High Ionization Energy Chlorine Has A Higher Ionization Energy Than Fluorine No, fluorine has a higher ionization energy than chlorine. Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes more energy to remove the electron from fluorine than in chlorine. Li < b < be < c < n < o < f < ne. Fluorine, though higher than chlorine in the periodic. Chlorine Has A Higher Ionization Energy Than Fluorine.
From byjus.com
Which has high ionization energy oxygen or chlorine?? Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. This is because the electrons in the outermost. No, fluorine has a higher ionization energy than chlorine. This makes the fluoride anion so. 120 rows ionization energy chart of all the elements is given below. Since the most loosely held electron. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.numerade.com
SOLVED Arrange these elements according to first ionization energy Chlorine Has A Higher Ionization Energy Than Fluorine This makes the fluoride anion so. This is because the electrons in the outermost. Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons are. Fluorine is the most electronegative element in the periodic table,. No, fluorine has a higher ionization energy than chlorine. Since the. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.numerade.com
SOLVED Rank the following elements according to their ionization Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes more energy to remove the electron from fluorine than in chlorine. Li < b < be < c < n < o < f. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.numerade.com
SOLVEDUsing an MO energylevel diagram, would you expect F2 to. have a Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. This makes the fluoride anion so. Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons are. This is because the electrons in the outermost. Fluorine is. Chlorine Has A Higher Ionization Energy Than Fluorine.
From byjus.com
Which has high ionization energy oxygen or chlorine?? Chlorine Has A Higher Ionization Energy Than Fluorine This is because the electrons in the outermost. Li < b < be < c < n < o < f < ne. This makes the fluoride anion so. 120 rows ionization energy chart of all the elements is given below. Fluorine is the most electronegative element in the periodic table,. No, fluorine has a higher ionization energy than chlorine.. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.geeksforgeeks.org
Ionization Energy Definition, Formulas, and Solved Examples Chlorine Has A Higher Ionization Energy Than Fluorine 120 rows ionization energy chart of all the elements is given below. Fluorine is the most electronegative element in the periodic table,. Fluorine, which is higher up the group then chlorine, has a lower electron affinity. Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons. Chlorine Has A Higher Ionization Energy Than Fluorine.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Chlorine Has A Higher Ionization Energy Than Fluorine Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes more energy to remove the electron from fluorine than in chlorine. Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. This is because the electrons in the outermost. Fluorine, though higher than chlorine. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine is the most electronegative element in the periodic table,. This is because the electrons in the outermost. Li < b < be < c < n < o < f < ne. Fluorine, which is higher up the group then chlorine, has a lower electron affinity. 120 rows ionization energy chart of all the elements is given below. Fluorine,. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.youtube.com
Chlorine has ___________ electron affinity than fluorine. YouTube Chlorine Has A Higher Ionization Energy Than Fluorine 120 rows ionization energy chart of all the elements is given below. No, fluorine has a higher ionization energy than chlorine. This is because the electrons in the outermost. Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons are. Li < b < be <. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.ck12.org
Periodic Trends in Ionization Energy CK12 Foundation Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons are. Fluorine is the most electronegative element in the periodic table,. Li < b < be < c < n < o < f < ne. No, fluorine has a higher ionization energy than chlorine. 120. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine, which is higher up the group then chlorine, has a lower electron affinity. This makes the fluoride anion so. Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons are. This is because the electrons in the outermost. First ionization energy, second ionization energy as. Chlorine Has A Higher Ionization Energy Than Fluorine.
From periodictable.me
Electron+Configuration_+Chlorine Dynamic Periodic Table of Elements Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons are. Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. Li < b < be < c < n < o < f < ne. Since. Chlorine Has A Higher Ionization Energy Than Fluorine.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine is the most electronegative element in the periodic table,. This is because the electrons in the outermost. This makes the fluoride anion so. Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. First ionization energy, second ionization energy as well as. Since the most loosely held electron in fluorine. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.periodic-table.org
Chlorine Ionization Energy Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons are. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Fluorine, which is higher up the group then chlorine, has a lower electron affinity. No, fluorine has a higher ionization. Chlorine Has A Higher Ionization Energy Than Fluorine.
From sciencenotes.org
What Is Ionization Energy? Definition and Trend Chlorine Has A Higher Ionization Energy Than Fluorine 120 rows ionization energy chart of all the elements is given below. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. First ionization energy, second ionization energy as well as. This is because the electrons in the outermost. Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it. Chlorine Has A Higher Ionization Energy Than Fluorine.
From chemistnotes.com
Periodic table Archives Chemistry Notes Chlorine Has A Higher Ionization Energy Than Fluorine No, fluorine has a higher ionization energy than chlorine. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. This is because the electrons in the outermost. 120 rows ionization energy chart of all the elements is given below. Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it. Chlorine Has A Higher Ionization Energy Than Fluorine.
From slideplayer.com
Learning Goals Understand the periodic trends ppt download Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes more energy to remove the electron from fluorine than. Chlorine Has A Higher Ionization Energy Than Fluorine.
From chemistry.stackexchange.com
periodic trends If fluorine has a lower electron affinity than Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Fluorine, which is higher up the group then chlorine, has a lower electron affinity. First ionization energy, second ionization energy as well as. No, fluorine has a higher ionization energy than chlorine. Fluorine is the most electronegative element in the periodic table,. Li < b. Chlorine Has A Higher Ionization Energy Than Fluorine.
From courses.lumenlearning.com
Periodic Variations in Element Properties Chemistry Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. First ionization energy, second ionization energy as well as. This is because the electrons in the outermost. Fluorine, which is higher up the group then chlorine, has a lower electron affinity. This makes the fluoride anion so. No, fluorine has a higher ionization energy than. Chlorine Has A Higher Ionization Energy Than Fluorine.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine is the most electronegative element in the periodic table,. Fluorine, which is higher up the group then chlorine, has a lower electron affinity. First ionization energy, second ionization energy as well as. This makes the fluoride anion so. Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. This is. Chlorine Has A Higher Ionization Energy Than Fluorine.
From brainly.in
Why chlorine has a higher electron affinity than fluorine? Brainly.in Chlorine Has A Higher Ionization Energy Than Fluorine No, fluorine has a higher ionization energy than chlorine. Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. Li < b < be < c < n < o < f < ne. First ionization energy, second ionization energy as well as. Since the most loosely held electron in fluorine. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.slideserve.com
PPT The Periodic Table & Its Properties Chapters 5 & 6 PowerPoint Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine is the most electronegative element in the periodic table,. Li < b < be < c < n < o < f < ne. 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. No, fluorine has a higher ionization energy than chlorine. This is because the electrons. Chlorine Has A Higher Ionization Energy Than Fluorine.
From exopnbdff.blob.core.windows.net
Does Chlorine Have A Higher Ionization Energy Than Aluminum at Beth Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons are. Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. Since. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.chemistrylearner.com
Periodic Trends Definition and Properties Chlorine Has A Higher Ionization Energy Than Fluorine 120 rows ionization energy chart of all the elements is given below. Li < b < be < c < n < o < f < ne. First ionization energy, second ionization energy as well as. Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.numerade.com
SOLVED Arrange these elements according to first ionization energy Chlorine Has A Higher Ionization Energy Than Fluorine Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes more energy to remove the electron from fluorine than in chlorine. Fluorine is the most electronegative element in the periodic table,. 120 rows ionization energy chart of all the elements is given below. Fluorine has a higher ionization energy than chlorine because it. Chlorine Has A Higher Ionization Energy Than Fluorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Chlorine Has A Higher Ionization Energy Than Fluorine 120 rows ionization energy chart of all the elements is given below. Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes more energy to remove the electron from fluorine than in chlorine. This is because the electrons in the outermost. Fluorine has a higher ionization energy than chlorine because it is higher. Chlorine Has A Higher Ionization Energy Than Fluorine.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons are. This is because the electrons in the outermost. Fluorine is the most electronegative element in the periodic table,. Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes. Chlorine Has A Higher Ionization Energy Than Fluorine.
From commons.wikimedia.org
FileIonization energy atomic size.svg Wikimedia Commons Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. This is because the electrons in the outermost. First ionization energy, second ionization energy as well as. Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. 120 rows ionization energy chart of all the elements. Chlorine Has A Higher Ionization Energy Than Fluorine.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Chlorine Has A Higher Ionization Energy Than Fluorine This is because the electrons in the outermost. Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes more energy to remove the electron from fluorine than in chlorine. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. 120 rows ionization energy chart of all the elements. Chlorine Has A Higher Ionization Energy Than Fluorine.
From chem.libretexts.org
7.4 Ionization Energy Chemistry LibreTexts Chlorine Has A Higher Ionization Energy Than Fluorine This makes the fluoride anion so. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Fluorine is more electronegative, has a smaller atomic radius, and has a higher ionization energy and electron affinity than. First ionization energy, second ionization energy as well as. Fluorine has a higher ionization energy than chlorine because it is. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.meritnation.com
Does chlorine have higher electron affinity value than fluorine Chlorine Has A Higher Ionization Energy Than Fluorine Since the most loosely held electron in fluorine is closer to the nucleus than chlorine, it takes more energy to remove the electron from fluorine than in chlorine. First ionization energy, second ionization energy as well as. This is because the electrons in the outermost. This makes the fluoride anion so. Fluorine is more electronegative, has a smaller atomic radius,. Chlorine Has A Higher Ionization Energy Than Fluorine.
From www.chegg.com
Solved E) chlorine has a greater ionization energy than Chlorine Has A Higher Ionization Energy Than Fluorine First ionization energy, second ionization energy as well as. Fluorine, which is higher up the group then chlorine, has a lower electron affinity. Fluorine is the most electronegative element in the periodic table,. No, fluorine has a higher ionization energy than chlorine. Li < b < be < c < n < o < f < ne. Fluorine, though higher. Chlorine Has A Higher Ionization Energy Than Fluorine.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Chlorine Has A Higher Ionization Energy Than Fluorine Fluorine, which is higher up the group then chlorine, has a lower electron affinity. Li < b < be < c < n < o < f < ne. This makes the fluoride anion so. Fluorine has a higher ionization energy than chlorine because it is higher up in the group on the periodic table, and its valence electrons are.. Chlorine Has A Higher Ionization Energy Than Fluorine.