Food Calorimetry Lab Report . You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. Analyze the various types of materials by which food are made of. Quantify the amount of chemical energy stored in different types of food (e.i. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. Use a calorimeter to determine the number of calories in 3 samples of food. Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other.
from www.studocu.com
Quantify the amount of chemical energy stored in different types of food (e.i. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. Use a calorimeter to determine the number of calories in 3 samples of food. Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. Analyze the various types of materials by which food are made of.
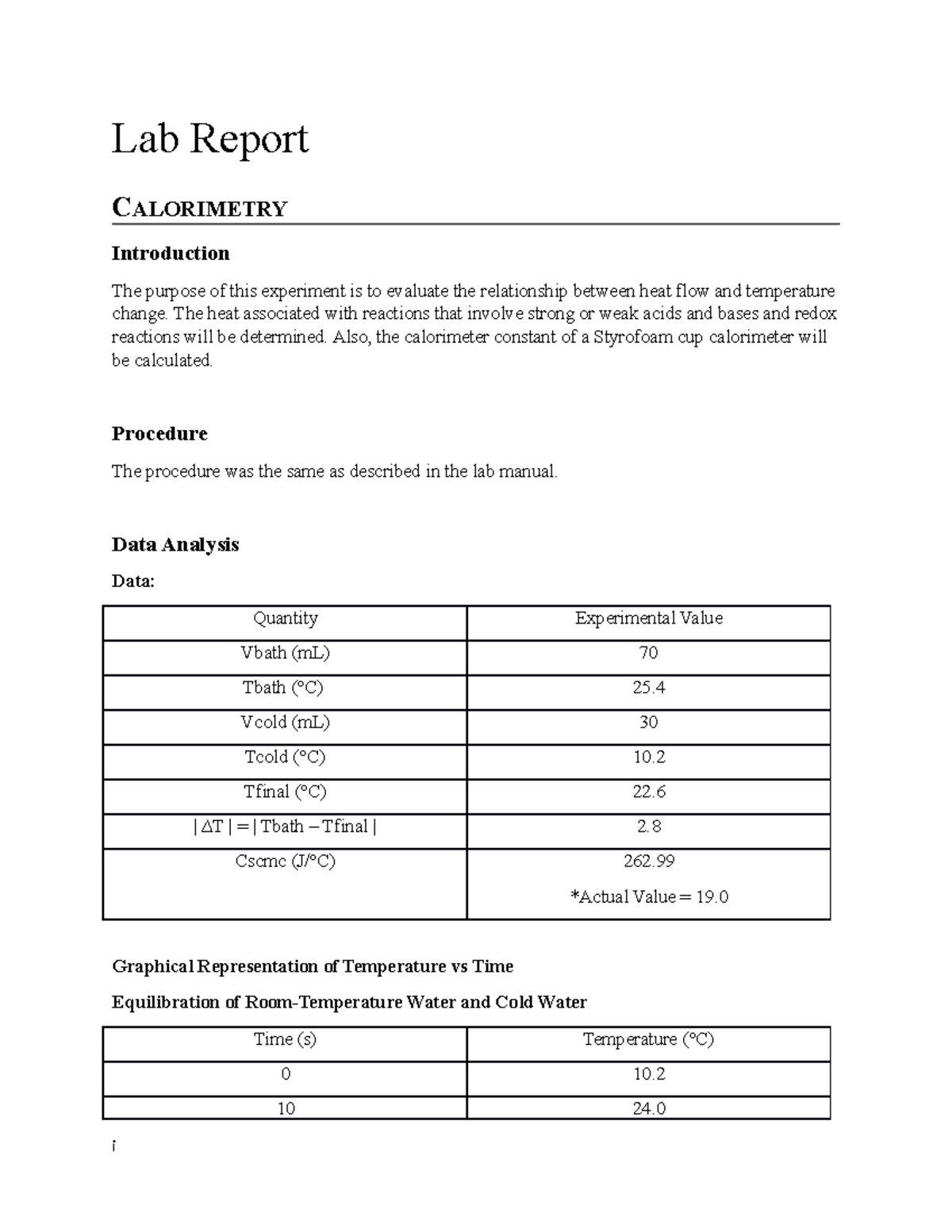
Lab Report Calorimetry Lab Report CALORIMETRY Introduction The purpose of this experiment is
Food Calorimetry Lab Report Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. Use a calorimeter to determine the number of calories in 3 samples of food. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. Quantify the amount of chemical energy stored in different types of food (e.i. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. Analyze the various types of materials by which food are made of. You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the.
From www.pdfprof.com
calorimetry experiment lab report conclusion Food Calorimetry Lab Report Analyze the various types of materials by which food are made of. Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. Quantify the amount of chemical energy stored in different types of food (e.i. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy. Food Calorimetry Lab Report.
From www.thinkswap.com
Calorimetry Lab Report PHYS1006 Foundations of Physics Curtin Thinkswap Food Calorimetry Lab Report Quantify the amount of chemical energy stored in different types of food (e.i. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. Use a calorimeter to determine the number of calories in 3 samples of food. You have been hired by a textbook publisher. Food Calorimetry Lab Report.
From www.youtube.com
Unit 8 Food Calorimetry Lab YouTube Food Calorimetry Lab Report From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. You have been hired by a textbook publisher to evaluate the. Food Calorimetry Lab Report.
From www.scribd.com
Calorimetry Lab SE PDF Calorie Heat Capacity Food Calorimetry Lab Report Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. You have been hired by a textbook publisher. Food Calorimetry Lab Report.
From www.pdfprof.com
determination of calorimeter constant lab report Food Calorimetry Lab Report Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. Use a calorimeter to determine the number of. Food Calorimetry Lab Report.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Calorimeter Studypool Food Calorimetry Lab Report Use a calorimeter to determine the number of calories in 3 samples of food. Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. From the measured temperature change,. Food Calorimetry Lab Report.
From materialdbtracy.z13.web.core.windows.net
Calorimetry Questions And Answers Food Calorimetry Lab Report You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted. Food Calorimetry Lab Report.
From pdfprof.com
experiment 25 calorimetry lab report Food Calorimetry Lab Report You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. Quantify the amount of chemical energy stored in different types of food (e.i. From the measured temperature. Food Calorimetry Lab Report.
From www.studocu.com
Lab Report Calorimetry Lab Report CALORIMETRY Introduction The purpose of this experiment is Food Calorimetry Lab Report Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. Analyze the various types of materials by which food are made of. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per. Food Calorimetry Lab Report.
From browsegrades.net
Lab Report >7.03 Calorimetry Lab Report_ Parkersburg South High School. Plus Q&A Browsegrades Food Calorimetry Lab Report Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. From. Food Calorimetry Lab Report.
From www.studocu.com
Calorimetry Lab Report (Part 1) For this experiment, the definition of calorimetry will be Food Calorimetry Lab Report You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. In this lab investigation, you will use the methods of calorimetry to approximate the. Food Calorimetry Lab Report.
From studylib.net
Lab Report Food Calorimetry Lab Report Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. Use a calorimeter to determine the number of calories in 3 samples of food. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. Quantify the amount of chemical energy. Food Calorimetry Lab Report.
From www.youtube.com
FOOD TESTS AND CALORIMETER EXPERIMENT YouTube Food Calorimetry Lab Report Use a calorimeter to determine the number of calories in 3 samples of food. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit. Food Calorimetry Lab Report.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Food Calorimetry Lab Report Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. Use a calorimeter to determine the number of calories in 3 samples of food. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. You have been hired by. Food Calorimetry Lab Report.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Food Calorimetry Lab Report Quantify the amount of chemical energy stored in different types of food (e.i. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram. Food Calorimetry Lab Report.
From studylib.net
06.03 Calorimetry Lab Report Food Calorimetry Lab Report Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. Use a calorimeter to determine the number of calories in 3 samples of food. You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for.. Food Calorimetry Lab Report.
From www.youtube.com
Energy in Foods Calorimetry Lab YouTube Food Calorimetry Lab Report Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. Use a calorimeter to determine the number of calories in 3 samples of food. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. From the. Food Calorimetry Lab Report.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry experiment. An example abstract Food Calorimetry Lab Report Use a calorimeter to determine the number of calories in 3 samples of food. Quantify the amount of chemical energy stored in different types of food (e.i. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. Construct a model to illustrate the flow of energy. Food Calorimetry Lab Report.
From www.studocu.com
Calorimetry Lab Report S Calorimetry Introduction In this experiment, the primary objective Food Calorimetry Lab Report Analyze the various types of materials by which food are made of. You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. Use a calorimeter to determine. Food Calorimetry Lab Report.
From www.slideshare.net
Calorimetry Lab Food Calorimetry Lab Report In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. Analyze the various types of materials by which food are made of. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram. Food Calorimetry Lab Report.
From studylib.net
Bomb Calorimeter Lab Sheet Food Calorimetry Lab Report You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. Analyze the various types of materials by which food are made of. From the. Food Calorimetry Lab Report.
From studylib.net
Student instructions and lab report Food Calorimetry Lab Report Analyze the various types of materials by which food are made of. You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. From the measured temperature change, students calculate the energy transferred to the water, and. Food Calorimetry Lab Report.
From www.studocu.com
Calorimetry Lab Report Name Mahma Ahmed Partner Wala Naeem Student No 20175600 Student No Food Calorimetry Lab Report Use a calorimeter to determine the number of calories in 3 samples of food. Quantify the amount of chemical energy stored in different types of food (e.i. Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine. Food Calorimetry Lab Report.
From www.carolina.com
Food Calorimetry How to Measure Calories in Food Food Calorimetry Lab Report Use a calorimeter to determine the number of calories in 3 samples of food. Analyze the various types of materials by which food are made of. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. From the measured temperature change, students. Food Calorimetry Lab Report.
From www.studocu.com
Calorimetry Lab Report Calorimetry Lab Report Introduction Calorimetry is a way to measure the Food Calorimetry Lab Report From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. Use a calorimeter to determine the number of calories in 3. Food Calorimetry Lab Report.
From www.scribd.com
lab 4 calorimetry lab Temperature Heat Capacity Food Calorimetry Lab Report Use a calorimeter to determine the number of calories in 3 samples of food. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. Analyze the various types of materials by which food are made of. Construct a model to illustrate the. Food Calorimetry Lab Report.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Calorimeter Studypool Food Calorimetry Lab Report You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. Quantify the amount of chemical energy stored in different types of food (e.i. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. Use a calorimeter to determine. Food Calorimetry Lab Report.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Docsity Food Calorimetry Lab Report You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. Analyze the. Food Calorimetry Lab Report.
From www.animalia-life.club
Food Calorimeter Food Calorimetry Lab Report In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. Use a calorimeter to determine the number of calories in 3. Food Calorimetry Lab Report.
From www.animalia-life.club
Food Calorimeter Food Calorimetry Lab Report From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. Analyze the various types of materials by which. Food Calorimetry Lab Report.
From www.studocu.com
Calorimetry pt. 1 lab report CALORIMETRY PART 1 S PECIFIC HEAT C APACITY LAB REPORT Name Food Calorimetry Lab Report Quantify the amount of chemical energy stored in different types of food (e.i. You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/ or other. Construct a model to. Food Calorimetry Lab Report.
From www.studocu.com
CoffeeCup Calorimetry Experiment Lab Report with answers and solutions LAB REPORT FOR Food Calorimetry Lab Report From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. Use a calorimeter to determine the number of calories in 3 samples of food. You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. Construct a model to. Food Calorimetry Lab Report.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Food Calorimetry Lab Report Quantify the amount of chemical energy stored in different types of food (e.i. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food. In this lab investigation, you will use the methods of calorimetry to approximate the amount of energy contained in a potato chip and/. Food Calorimetry Lab Report.
From www.docsity.com
Food Calorimetry Lab Lecture notes Law Docsity Food Calorimetry Lab Report Analyze the various types of materials by which food are made of. Quantify the amount of chemical energy stored in different types of food (e.i. Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present. Food Calorimetry Lab Report.
From www.scribd.com
Food Calorimetry Lab Report PDF Food Calorimetry Lab Report Analyze the various types of materials by which food are made of. Use the macronutrients (product reported quantities of carbohydrates, fat, and protein) of each food item to determine the energy content (kcal) per gram of the combusted foods and. You have been hired by a textbook publisher to evaluate the accuracy and feasibility of laboratory experiments for. From the. Food Calorimetry Lab Report.