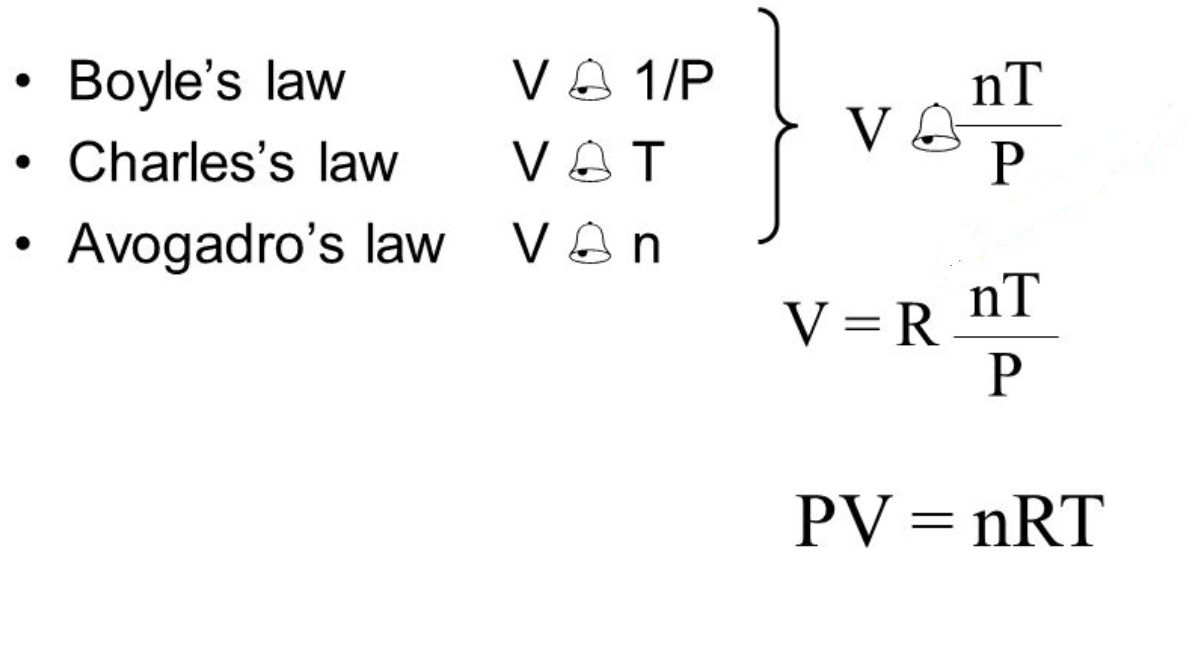
Gas Pressure Laws . Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. Gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and pressure associated with a. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Learn and apply boyle’s law. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. At constant temperature, the volume of a fixed amount. Examine kinetic energy and speed histograms for light and heavy. Learn what is meant by the term gas laws. Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful because it links pressure, density,. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Gas laws, laws that relate the pressure, volume, and temperature of a gas.
from scienceforyou.netlify.app
Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful because it links pressure, density,. Examine kinetic energy and speed histograms for light and heavy. Learn and apply boyle’s law. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Learn what is meant by the term gas laws. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. At constant temperature, the volume of a fixed amount. Gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and pressure associated with a. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows:
Charles and boyles law scienceforyou
Gas Pressure Laws Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Learn what is meant by the term gas laws. Learn and apply boyle’s law. Examine kinetic energy and speed histograms for light and heavy. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful because it links pressure, density,. Gas laws, laws that relate the pressure, volume, and temperature of a gas. At constant temperature, the volume of a fixed amount. Gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and pressure associated with a. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies.
From www.youtube.com
11.6 The Combined Gas Law Pressure, Volume, & Temperature YouTube Gas Pressure Laws Learn what is meant by the term gas laws. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. At. Gas Pressure Laws.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Presentation ID6855999 Gas Pressure Laws Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Learn what is meant by the term gas laws. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. At constant temperature, the volume of a fixed amount. Learn and apply boyle’s law.. Gas Pressure Laws.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Gas Pressure Laws Gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and pressure associated with a. Examine kinetic energy and speed histograms for light and heavy. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: The three fundamental gas. Gas Pressure Laws.
From owlcation.com
The Theories and Behavior of Gas Owlcation Gas Pressure Laws Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful because it links pressure, density,. Learn and apply boyle’s law. Gas laws, laws that relate the pressure, volume, and temperature. Gas Pressure Laws.
From inspiritvr.com
Combined Gas Law Study Guide Inspirit Learning Inc Gas Pressure Laws The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Learn and apply boyle’s law. At constant temperature, the volume of a fixed amount. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. This relationship between pressure and volume is known as boyle’s law, after. Gas Pressure Laws.
From www.britannica.com
Boyle’s law Definition, Equation, & Facts Britannica Gas Pressure Laws Gas laws, laws that relate the pressure, volume, and temperature of a gas. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. At constant temperature, the volume of a fixed amount. Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful. Gas Pressure Laws.
From www.clippard.com
Boyle's Law Clippard Knowledgebase Gas Pressure Laws Gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and pressure associated with a. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. Gas laws, laws that relate the pressure, volume, and temperature of a gas. At constant temperature,. Gas Pressure Laws.
From www.youtube.com
Combined Gas Law Pressure, Volume and Temperature Straight Science YouTube Gas Pressure Laws The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Learn and apply boyle’s law. Examine kinetic energy and speed histograms for light and heavy. Measure the temperature and pressure, and discover how the properties of the gas vary in relation. Gas Pressure Laws.
From scienceducatio.weebly.com
Gas Laws Environmental Chemistry Gas Pressure Laws This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Learn what is meant by the term gas. Gas Pressure Laws.
From sciencenotes.org
Boyle's Law Definition, Formula, Example Gas Pressure Laws At constant temperature, the volume of a fixed amount. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and pressure associated with a. Gas laws, laws that relate. Gas Pressure Laws.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii Gas Pressure Laws Gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and pressure associated with a. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. At constant temperature, the volume of a fixed amount. This relationship between pressure and volume is. Gas Pressure Laws.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 Gas Pressure Laws The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Examine kinetic energy and speed histograms for light and heavy. At constant temperature, the volume of a fixed amount. Learn and apply boyle’s law.. Gas Pressure Laws.
From www.youtube.com
Calculating Gas Pressure Pressure in the Gas Laws CLEAR & SIMPLE YouTube Gas Pressure Laws Learn what is meant by the term gas laws. Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful because it links pressure, density,. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Learn and apply boyle’s law. Gas laws are a group of. Gas Pressure Laws.
From study.com
GayLussac's Law Gas Pressure and Temperature Relationship Video & Lesson Transcript Gas Pressure Laws Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful because it links pressure, density,. Examine kinetic energy and speed histograms for light and heavy. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. Learn what is meant by. Gas Pressure Laws.
From scienceforyou.netlify.app
Charles and boyles law scienceforyou Gas Pressure Laws Gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and pressure associated with a. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Examine kinetic. Gas Pressure Laws.
From general.chemistrysteps.com
The Ideal Gas Law Chemistry Steps Gas Pressure Laws Learn and apply boyle’s law. Gas laws, laws that relate the pressure, volume, and temperature of a gas. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful. Gas Pressure Laws.
From sciencenotes.org
Ideal Gas Law Formula and Examples Gas Pressure Laws At constant temperature, the volume of a fixed amount. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Defining the specific gas constant rspecific as the ratio r / m, this form of the. Gas Pressure Laws.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Presentation ID6855999 Gas Pressure Laws At constant temperature, the volume of a fixed amount. Examine kinetic energy and speed histograms for light and heavy. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. Gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and pressure. Gas Pressure Laws.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 Gas Pressure Laws Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful because it links. Gas Pressure Laws.
From owlcation.com
The Theories and Behavior of Gas Owlcation Gas Pressure Laws At constant temperature, the volume of a fixed amount. Learn and apply boyle’s law. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Examine kinetic energy and speed histograms for light and heavy. Defining the specific gas constant rspecific as the ratio r / m, this form of the. Gas Pressure Laws.
From sciencenotes.org
Dalton's Law of Partial Pressure Definition and Examples Gas Pressure Laws Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful because it links pressure, density,. At constant temperature, the volume of a fixed amount. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Boyle’s law —named for robert. Gas Pressure Laws.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal gas constant and Gas Pressure Laws Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful because it links pressure, density,. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Gas laws, laws that relate the pressure, volume, and temperature of a gas. This relationship between pressure. Gas Pressure Laws.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID3344909 Gas Pressure Laws Examine kinetic energy and speed histograms for light and heavy. Gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and pressure associated with a. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Boyle’s law —named for robert boyle —states that,. Gas Pressure Laws.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Presentation ID6855999 Gas Pressure Laws At constant temperature, the volume of a fixed amount. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful because it links pressure, density,. Boyle’s law —named for robert. Gas Pressure Laws.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Gas Pressure Laws Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful because it links pressure, density,. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Examine kinetic energy and speed histograms for light and heavy. Gas laws, laws that. Gas Pressure Laws.
From fineartamerica.com
Gay Lussac's Pressuretemperature Gas Law Photograph by Science Photo Library Fine Art America Gas Pressure Laws Examine kinetic energy and speed histograms for light and heavy. Learn and apply boyle’s law. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. The three fundamental gas laws discover the. Gas Pressure Laws.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID5864985 Gas Pressure Laws Gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and pressure associated with a. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Learn and apply boyle’s law. At constant temperature, the volume of a fixed amount. Measure the temperature and. Gas Pressure Laws.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock Vector Illustration of Gas Pressure Laws At constant temperature, the volume of a fixed amount. Learn and apply boyle’s law. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Examine kinetic energy and speed histograms for light and heavy.. Gas Pressure Laws.
From studiousguy.com
The Gas Laws Definition, Formula & Examples StudiousGuy Gas Pressure Laws Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Learn what is meant by the term gas laws. Examine kinetic energy and speed histograms for light and heavy. Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful. Gas Pressure Laws.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Presentation ID3207761 Gas Pressure Laws Learn and apply boyle’s law. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Learn what is meant by the term gas laws. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal. Gas Pressure Laws.
From med.libretexts.org
2.3 Gaseous Exchange Mechanism Medicine LibreTexts Gas Pressure Laws Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Learn what is meant by the term gas laws. Gas laws are a group of laws that govern the behaviour of gases by providing relationships between the temperature, moles, volume and pressure associated with a. Learn and apply boyle’s law. Boyle’s. Gas Pressure Laws.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii Gas Pressure Laws Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Learn and apply boyle’s law. Learn what is meant by the term gas laws. Defining the specific gas constant rspecific as the ratio r / m, this form. Gas Pressure Laws.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Presentation ID3207761 Gas Pressure Laws Learn what is meant by the term gas laws. Boyle’s law —named for robert boyle —states that, at constant temperature, the pressure p of a gas varies. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to. Gas Pressure Laws.
From studiousguy.com
8 Boyle’s Law Examples in Real Life StudiousGuy Gas Pressure Laws Learn and apply boyle’s law. The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Defining the specific gas constant rspecific as. Gas Pressure Laws.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica Gas Pressure Laws Defining the specific gas constant rspecific as the ratio r / m, this form of the ideal gas law is very useful because it links pressure, density,. Examine kinetic energy and speed histograms for light and heavy. At constant temperature, the volume of a fixed amount. Gas laws are a group of laws that govern the behaviour of gases by. Gas Pressure Laws.