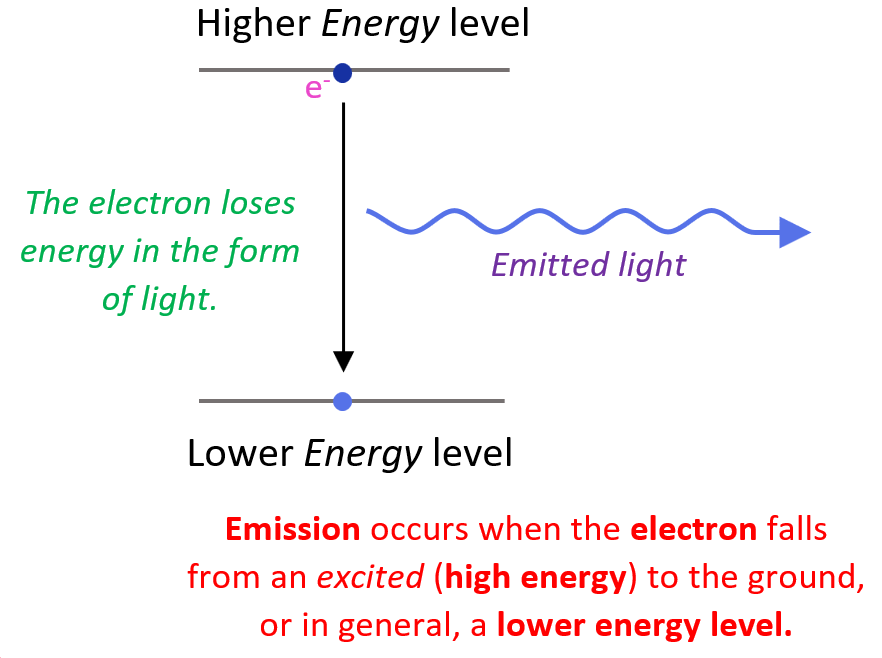
Electron In Hydrogen Atom . bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure. the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. Bohr was clever enough to find a. the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has the minimum possible quantity of energy. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons.
from general.chemistrysteps.com
the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has the minimum possible quantity of energy. Bohr was clever enough to find a. the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition.
Bohr Model of the Hydrogen Atom Chemistry Steps
Electron In Hydrogen Atom bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure. the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has the minimum possible quantity of energy. bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Bohr was clever enough to find a. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition.
From homedeso.vercel.app
Hydrogen Periodic Table Electron In Hydrogen Atom bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure. the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen. Electron In Hydrogen Atom.
From stock.adobe.com
Diagram of a hydrogen atom showing a proton in the nucleus and an Electron In Hydrogen Atom the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. when an electron in a hydrogen atom is in the first. Electron In Hydrogen Atom.
From www.sciencephoto.com
Hydrogen, atomic structure Stock Image C018/3682 Science Photo Library Electron In Hydrogen Atom Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr. Electron In Hydrogen Atom.
From www.dreamstime.com
Atom of Hydrogen with Detailed Core and Its Electron with Atoms Stock Electron In Hydrogen Atom Bohr was clever enough to find a. the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has. Electron In Hydrogen Atom.
From www.youtube.com
Bohr Model of the Hydrogen Atom YouTube Electron In Hydrogen Atom bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. the distribution of electron density predicted by the solution of schrödinger’s equation. Electron In Hydrogen Atom.
From www.alamy.com
Bohr Model Of Scientific Hydrogen Atom Vector. Structure Nucleus Of Electron In Hydrogen Atom when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has the minimum possible quantity of energy. in this section we will discuss the energy. Electron In Hydrogen Atom.
From www.wikihow.com
How to Learn About the Chemistry of the Hydrogen Atom 12 Steps Electron In Hydrogen Atom the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. the hydrogen atom consists of a single negatively charged electron that moves. Electron In Hydrogen Atom.
From mungfali.com
Hydrogen Atomic Structure Electron In Hydrogen Atom Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. Bohr was clever enough to find a. the hydrogen atom is the simplest atom in nature and, therefore,. Electron In Hydrogen Atom.
From periodictable.me
How Can You Find The Hydrogen Electron Configuration (H) Electron In Hydrogen Atom the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has the minimum possible quantity of energy. in this section we will discuss the energy level of the electron. Electron In Hydrogen Atom.
From depositphotos.com
Render Atom Structure Hydrogen Isolated White Backgroun Stock Photo by Electron In Hydrogen Atom bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure. the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen. Electron In Hydrogen Atom.
From www.vectorstock.com
Symbol and electron diagram for hydrogen Vector Image Electron In Hydrogen Atom when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. Bohr’s model combines the classical mechanics of planetary motion with the quantum. Electron In Hydrogen Atom.
From fineartamerica.com
Art Of Hydrogen Atom With Electron In Orbital Photograph by Laguna Design Electron In Hydrogen Atom the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has the minimum possible quantity of energy. when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. bohr’s model of the hydrogen atom, proposed by. Electron In Hydrogen Atom.
From valenceelectrons.com
Hydrogen Electron Configuration And Full Orbital Diagram Electron In Hydrogen Atom Bohr was clever enough to find a. when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. Bohr’s model combines the classical. Electron In Hydrogen Atom.
From www.youtube.com
Energy level diagram of an electron in the Hydrogen atom part2 YouTube Electron In Hydrogen Atom the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has the minimum possible quantity of energy. Bohr was clever enough to find a. the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure. Bohr’s model combines the classical mechanics of planetary motion. Electron In Hydrogen Atom.
From www.naturphilosophie.co.uk
At the Heart of the Hydrogen Atom... NaturPhilosophie Electron In Hydrogen Atom Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Bohr was clever enough to find a. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. when an electron in a hydrogen atom is in the first. Electron In Hydrogen Atom.
From www.alamy.com
Hydrogen atom diagram concept Stock Vector Image & Art Alamy Electron In Hydrogen Atom Bohr was clever enough to find a. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. the hydrogen atom is the simplest atom in nature and, therefore,. Electron In Hydrogen Atom.
From hydrogenatomgirikosa.blogspot.com
Hydrogen Atom You Are The Most Important Electron In A Hydrogen Atom Electron In Hydrogen Atom when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. Bohr was clever enough to find a. the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has the minimum possible quantity of energy. bohr’s. Electron In Hydrogen Atom.
From userdatasynoecizes.z5.web.core.windows.net
Energy Diagram Of Hydrogen Atom Electron In Hydrogen Atom Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure. the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has the minimum possible quantity of energy. . Electron In Hydrogen Atom.
From www.vecteezy.com
Vector of Hydrogen atom. Hydrogen atom with atomic mass , atomic number Electron In Hydrogen Atom the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure. the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr. Electron In Hydrogen Atom.
From www.dreamstime.com
Hydrogen Atom Bohr Model with Proton, Neutron and Electron Stock Electron In Hydrogen Atom when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. the hydrogen atom consists of a single negatively charged electron that. Electron In Hydrogen Atom.
From www.dreamstime.com
Diagram Representation of the Element Hydrogen Stock Vector Electron In Hydrogen Atom Bohr was clever enough to find a. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has the minimum possible quantity of energy. the hydrogen atom consists of a single negatively charged electron that moves. Electron In Hydrogen Atom.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Electron In Hydrogen Atom in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. the hydrogen atom is the simplest atom in. Electron In Hydrogen Atom.
From homepagetop.com
Hydrogen(H) electron configuration and orbital diagram (2023) Electron In Hydrogen Atom Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure. the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has the minimum possible quantity of energy. Bohr. Electron In Hydrogen Atom.
From byjus.com
Uses Of Hydrogen Properties & its Reactions with Videos Electron In Hydrogen Atom when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. the distribution of electron density predicted by the. Electron In Hydrogen Atom.
From www.reddit.com
Hydrogen Orbitals r/chemistry Electron In Hydrogen Atom the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure. when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. Bohr was clever enough to find a. Bohr’s model combines the classical mechanics of planetary. Electron In Hydrogen Atom.
From periodictable.me
How Can You Find The Hydrogen Electron Configuration (H) Electron In Hydrogen Atom bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has the minimum possible quantity of energy. Bohr’s model combines the classical mechanics of planetary motion with. Electron In Hydrogen Atom.
From animalia-life.club
Bohr Atomic Model Of Hydrogen Electron In Hydrogen Atom when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure.. Electron In Hydrogen Atom.
From www.alamy.com
Hydrogen Atom Bohr model with proton and electron. 3d illustration Electron In Hydrogen Atom the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. the distribution of. Electron In Hydrogen Atom.
From periodictable.me
Valence Electrons in a Hydrogen Atom Archives Dynamic Periodic Table Electron In Hydrogen Atom Bohr was clever enough to find a. bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. the distribution of electron density predicted by the solution of schrödinger’s equation. Electron In Hydrogen Atom.
From www.alamy.com
Hydrogen atom showing a single electron orbiting a single proton Stock Electron In Hydrogen Atom when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. Bohr was clever enough to find a. the distribution of electron density predicted by the solution of schrödinger’s equation for a hydrogen atom which has the minimum possible quantity of energy. the. Electron In Hydrogen Atom.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Electron In Hydrogen Atom Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr model give about its orbital speed and. the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms. Electron In Hydrogen Atom.
From hydrogenatomgirikosa.blogspot.com
Hydrogen Atom Distance Between Proton And Electron In Hydrogen Atom Electron In Hydrogen Atom Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Bohr was clever enough to find a. the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton (figure. in this section we will discuss the energy level of the electron of a hydrogen atom, and how. Electron In Hydrogen Atom.
From www.vectorstock.com
Diagram representation element hydrogen Royalty Free Vector Electron In Hydrogen Atom Bohr was clever enough to find a. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. the hydrogen atom consists of a single negatively charged electron that moves. Electron In Hydrogen Atom.
From chemwiki.ucdavis.edu
9 The Hydrogen Atom Chemwiki Electron In Hydrogen Atom in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms. Electron In Hydrogen Atom.
From pixabay.com
Hydrogen Atom · Free vector graphic on Pixabay Electron In Hydrogen Atom in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. when an electron in a hydrogen atom is in the first excited state, what prediction does the bohr. Electron In Hydrogen Atom.