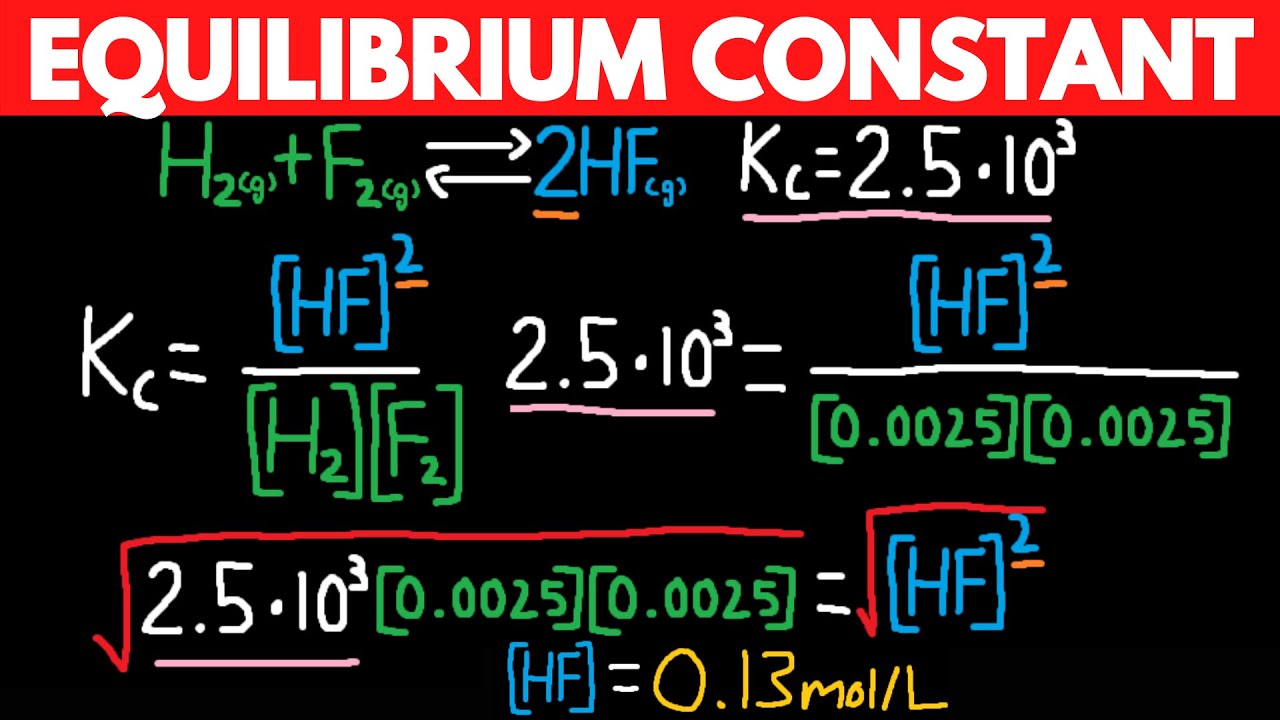
How To Calculate Kc From Other Kc . Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. You might be expected to calculate a value for k c including its units (which vary from case to case). Alternatively you might have to calculate equilibrium concentrations from a given. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. The key to solving this problem is to. Use the coefficients in the balanced chemical equation to obtain the changes in concentration of all other substances in the. 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Use the coefficients in the balanced chemical equation to calculate \ (δn\). Calculate the value of k c for the reaction: Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\).
from printablelibscapus.z21.web.core.windows.net
Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. The key to solving this problem is to. Alternatively you might have to calculate equilibrium concentrations from a given. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Use the coefficients in the balanced chemical equation to calculate \ (δn\). Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Calculate the value of k c for the reaction: You might be expected to calculate a value for k c including its units (which vary from case to case). Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). Use the coefficients in the balanced chemical equation to obtain the changes in concentration of all other substances in the.
How To Calculate Kc Equilibrium Constant
How To Calculate Kc From Other Kc Alternatively you might have to calculate equilibrium concentrations from a given. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. You might be expected to calculate a value for k c including its units (which vary from case to case). Alternatively you might have to calculate equilibrium concentrations from a given. 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Calculate the value of k c for the reaction: The key to solving this problem is to. Use the coefficients in the balanced chemical equation to obtain the changes in concentration of all other substances in the. Use the coefficients in the balanced chemical equation to calculate \ (δn\).
From www.pinterest.co.kr
Learn how to calculate an equilibrium constant Kc. Teaching chemistry How To Calculate Kc From Other Kc 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). The key to solving this problem is to. You might be expected to calculate a value for k c including its units (which vary from case to case). Use the. How To Calculate Kc From Other Kc.
From www.youtube.com
Equilibrium Constants How to Calculate Kp from Kc and Vice Versa YouTube How To Calculate Kc From Other Kc 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. You might be expected to calculate a value for k c including its units (which vary from case to case). Use the coefficients in the balanced chemical equation to obtain the changes in concentration of all other substances in the. The. How To Calculate Kc From Other Kc.
From www.youtube.com
Calculating Kc From Experiments YouTube How To Calculate Kc From Other Kc 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Calculate the value of k c for the reaction: You might be expected to calculate a value for k c including its units (which vary from case to case). Use the coefficients in the balanced chemical equation to obtain the changes. How To Calculate Kc From Other Kc.
From www.youtube.com
R2.3.6 Calculating equilibrium concentrations from Kc and initial How To Calculate Kc From Other Kc Alternatively you might have to calculate equilibrium concentrations from a given. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). Calculate the value of k c for the reaction: You might be expected to calculate a value for k c. How To Calculate Kc From Other Kc.
From www.pinterest.com
Learn how the equilibrium constants Kc and Kp are related. Chemistry How To Calculate Kc From Other Kc Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. The key to solving this problem is to. You might be expected to calculate a value for k c including its units (which vary from case to. How To Calculate Kc From Other Kc.
From haipernews.com
How To Calculate Kc Given Kp Haiper How To Calculate Kc From Other Kc Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Calculate the value of k c for the reaction: Use the coefficients in the balanced chemical equation to calculate \ (δn\). Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). Write out the balanced chemical equation with the concentrations of beneath each. How To Calculate Kc From Other Kc.
From www.youtube.com
How to predict the direction of equilibrium by comparing Kc and Qc How To Calculate Kc From Other Kc You might be expected to calculate a value for k c including its units (which vary from case to case). The key to solving this problem is to. Alternatively you might have to calculate equilibrium concentrations from a given. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. 2 n 2 o(g) + 3. How To Calculate Kc From Other Kc.
From www.slideserve.com
PPT Calculating K c values PowerPoint Presentation ID6822447 How To Calculate Kc From Other Kc Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. You might be expected to calculate a value for k. How To Calculate Kc From Other Kc.
From haipernews.com
How To Calculate Kc Expression Haiper How To Calculate Kc From Other Kc The key to solving this problem is to. Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). Use the coefficients in the balanced chemical equation to obtain the changes in concentration of all other substances in the. Use the coefficients in the balanced chemical equation to calculate \ (δn\). Calculate the value of k c for the reaction:. How To Calculate Kc From Other Kc.
From www.youtube.com
Calculate Kc from Kp and vice versa YouTube How To Calculate Kc From Other Kc Calculate the value of k c for the reaction: 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Alternatively you might have to calculate equilibrium concentrations from a given. The key to. How To Calculate Kc From Other Kc.
From www.youtube.com
How to calculate kc value? YouTube How To Calculate Kc From Other Kc Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). Use the coefficients in the balanced chemical equation to obtain the changes in concentration of all other substances in the. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Calculate the product of equilibrium concentrations of reactants (raised to their. How To Calculate Kc From Other Kc.
From www.youtube.com
How to Calculate Kc and Kp Chemistry Equilibrium Practice Problems How To Calculate Kc From Other Kc Alternatively you might have to calculate equilibrium concentrations from a given. Calculate the value of k c for the reaction: Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). Write out the balanced chemical equation with the concentrations of beneath each substance using. How To Calculate Kc From Other Kc.
From haipernews.com
How To Find Kc If Kp Is Given Haiper How To Calculate Kc From Other Kc Use the coefficients in the balanced chemical equation to calculate \ (δn\). Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Alternatively you might have to calculate equilibrium concentrations from a given. Write out the balanced chemical equation. How To Calculate Kc From Other Kc.
From haipernews.com
How To Calculate Kc From Equilibrium Concentrations Haiper How To Calculate Kc From Other Kc 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Use the coefficients in the balanced chemical equation to calculate \ (δn\). Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). You might be expected to calculate a value for k c including its units (which vary from case. How To Calculate Kc From Other Kc.
From www.youtube.com
Week 6 15. Calculating Kc from Kp YouTube How To Calculate Kc From Other Kc Alternatively you might have to calculate equilibrium concentrations from a given. You might be expected to calculate a value for k c including its units (which vary from case to case). The key to solving this problem is to. Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). Calculate the product of equilibrium concentrations of reactants (raised to. How To Calculate Kc From Other Kc.
From haipernews.com
How To Calculate Kc With An Ice Table Haiper How To Calculate Kc From Other Kc Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. The key to solving this problem is to. Calculate the value of k c for the reaction: Write out the balanced chemical equation with the concentrations of beneath each. How To Calculate Kc From Other Kc.
From www.youtube.com
Kc Calculations YouTube How To Calculate Kc From Other Kc Use the coefficients in the balanced chemical equation to obtain the changes in concentration of all other substances in the. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Then use equation 15.3.3 to calculate. How To Calculate Kc From Other Kc.
From haipernews.com
How To Calculate Kc Given Concentration Haiper How To Calculate Kc From Other Kc The key to solving this problem is to. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Use the coefficients in the balanced chemical equation to calculate \ (δn\). Calculate \(k\) for. How To Calculate Kc From Other Kc.
From www.youtube.com
Calculating the Equilibrium Concentrations Using the Quadratic Formula How To Calculate Kc From Other Kc Calculate the value of k c for the reaction: Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. You might be expected to calculate a value for k c including its units (which vary. How To Calculate Kc From Other Kc.
From haipernews.com
How To Calculate Kc Haiper How To Calculate Kc From Other Kc Alternatively you might have to calculate equilibrium concentrations from a given. The key to solving this problem is to. Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Write out the balanced chemical equation with the concentrations of. How To Calculate Kc From Other Kc.
From www.youtube.com
Find Kp from Kc YouTube How To Calculate Kc From Other Kc 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Alternatively you might have to calculate equilibrium concentrations from a given. Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). You might be. How To Calculate Kc From Other Kc.
From haipernews.com
How To Calculate Kc With Kp Haiper How To Calculate Kc From Other Kc Use the coefficients in the balanced chemical equation to calculate \ (δn\). You might be expected to calculate a value for k c including its units (which vary from case to case). Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change. How To Calculate Kc From Other Kc.
From www.youtube.com
How to Calculate Kc and Its Units Chemical Equilibrium YouTube How To Calculate Kc From Other Kc Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. The key to solving this problem is to. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). Alternatively you might have to calculate equilibrium concentrations from a. How To Calculate Kc From Other Kc.
From lessonschoolintimistes.z14.web.core.windows.net
How To Calculate Kc Equilibrium Constant How To Calculate Kc From Other Kc Use the coefficients in the balanced chemical equation to obtain the changes in concentration of all other substances in the. You might be expected to calculate a value for k c including its units (which vary from case to case). Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Alternatively you. How To Calculate Kc From Other Kc.
From www.youtube.com
Calculating Kc from Kp and vice versa YouTube How To Calculate Kc From Other Kc Use the coefficients in the balanced chemical equation to calculate \ (δn\). Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Calculate the value of k c for the reaction: Then use equation 15.3.3 to. How To Calculate Kc From Other Kc.
From www.vrogue.co
Kp And Kc Relationship Chemistry Steps vrogue.co How To Calculate Kc From Other Kc The key to solving this problem is to. Use the coefficients in the balanced chemical equation to calculate \ (δn\). You might be expected to calculate a value for k c including its units (which vary from case to case). Calculate the value of k c for the reaction: Write out the balanced chemical equation with the concentrations of beneath. How To Calculate Kc From Other Kc.
From general.chemistrysteps.com
Kp and Kc Relationship Chemistry Steps How To Calculate Kc From Other Kc Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Use the coefficients in the balanced chemical equation to obtain the changes in concentration of all other substances in the. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Use the coefficients in the balanced. How To Calculate Kc From Other Kc.
From www.thesciencehive.co.uk
Chemical equilibrium* — the science sauce How To Calculate Kc From Other Kc Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Alternatively you might have to calculate equilibrium concentrations from a given. 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Calculate the value of k c for the reaction: Then use equation 15.3.3 to. How To Calculate Kc From Other Kc.
From calculatorshub.net
KCcalculator van KP Online How To Calculate Kc From Other Kc Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Calculate the value of k c for the reaction: 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Use the coefficients in the balanced chemical equation to calculate \ (δn\). The key. How To Calculate Kc From Other Kc.
From printablelibscapus.z21.web.core.windows.net
How To Calculate Kc Equilibrium Constant How To Calculate Kc From Other Kc 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Calculate the value of k c for the reaction: Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Alternatively you might have to calculate equilibrium concentrations from a given. You might be. How To Calculate Kc From Other Kc.
From www.chemistrystudent.com
Calculating Equilibrium Constant, Kc (ALevel) ChemistryStudent How To Calculate Kc From Other Kc Calculate the value of k c for the reaction: Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Alternatively you might have to calculate equilibrium concentrations from a given. Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o. How To Calculate Kc From Other Kc.
From materialoster.z21.web.core.windows.net
How To Calculate Kc Equilibrium Constant How To Calculate Kc From Other Kc The key to solving this problem is to. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Then use equation 15.3.3 to calculate \ (k\) from \ (k_p\). Alternatively you might have to calculate equilibrium concentrations from a given. Use the coefficients in the balanced chemical equation to calculate \ (δn\).. How To Calculate Kc From Other Kc.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc Membership How To Calculate Kc From Other Kc Calculate the value of k c for the reaction: Calculate \(k\) for the overall equation by multiplying the equilibrium constants for the individual equations. Alternatively you might have to calculate equilibrium concentrations from a given. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Write out the balanced chemical equation with the concentrations. How To Calculate Kc From Other Kc.
From www.youtube.com
How to calculate kc in equilibrium? YouTube How To Calculate Kc From Other Kc 2 n 2 o(g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Calculate the value of k c for the reaction: Use the coefficients in the balanced chemical equation to obtain the changes in concentration. How To Calculate Kc From Other Kc.
From haipernews.com
How To Calculate Kc Given Concentration Haiper How To Calculate Kc From Other Kc Use the coefficients in the balanced chemical equation to obtain the changes in concentration of all other substances in the. Calculate the product of equilibrium concentrations of reactants (raised to their coefficients in the balanced chemical. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. The key to solving this problem. How To Calculate Kc From Other Kc.