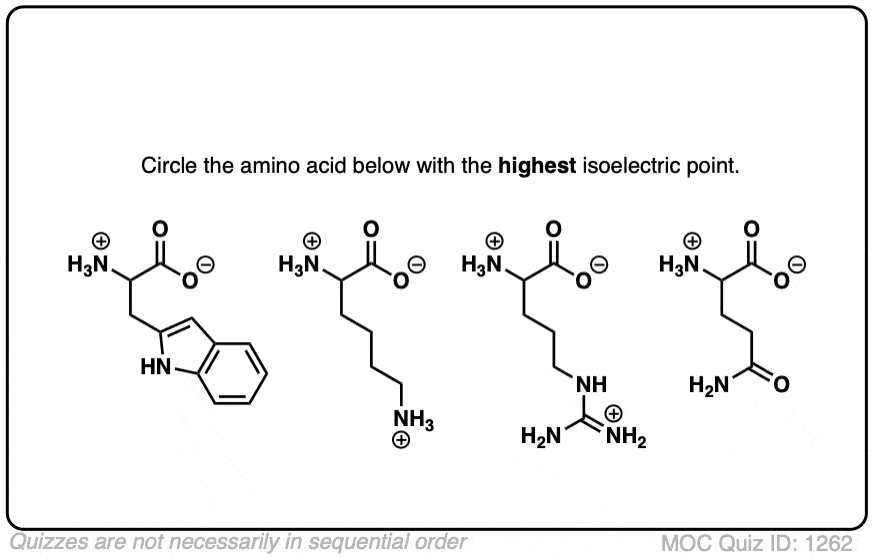
What Is The Isoelectric Point Of Lysine . The isoelectric point (ip) is the ph at which the amino acid has an overall zero charge the isoelectric points (ip) of amino acids range. If an amino acid has only one amino acid and only one carboxyl group, then the isoelectric point pi is calculated. Define zwitterion and isoelectric point. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average. In other words, the positively charged groups are. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. Determine the charge on an amino acid when it is not at the isoelectric point. An aminoacid carrying no net charge is called. Label amino acids as polar and nonpolar and as acidic, basic, or neutral.
from www.masterorganicchemistry.com
Define zwitterion and isoelectric point. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. In other words, the positively charged groups are. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. Determine the charge on an amino acid when it is not at the isoelectric point. An aminoacid carrying no net charge is called. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average. The isoelectric point (ip) is the ph at which the amino acid has an overall zero charge the isoelectric points (ip) of amino acids range. If an amino acid has only one amino acid and only one carboxyl group, then the isoelectric point pi is calculated. Label amino acids as polar and nonpolar and as acidic, basic, or neutral.
Isoelectric Points of Amino Acids (and How To Calculate Them) Master
What Is The Isoelectric Point Of Lysine If an amino acid has only one amino acid and only one carboxyl group, then the isoelectric point pi is calculated. Determine the charge on an amino acid when it is not at the isoelectric point. If an amino acid has only one amino acid and only one carboxyl group, then the isoelectric point pi is calculated. In other words, the positively charged groups are. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average. Define zwitterion and isoelectric point. An aminoacid carrying no net charge is called. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. Label amino acids as polar and nonpolar and as acidic, basic, or neutral. The isoelectric point (ip) is the ph at which the amino acid has an overall zero charge the isoelectric points (ip) of amino acids range. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge.
From www.youtube.com
B.2 Isoelectric point of amino acids (SL) YouTube What Is The Isoelectric Point Of Lysine In other words, the positively charged groups are. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average. Define zwitterion and isoelectric point. Label amino acids as polar and nonpolar and as acidic, basic, or neutral. Determine the charge on an amino acid when it is not. What Is The Isoelectric Point Of Lysine.
From www3.nd.edu
Amino Acid Titration Curves What Is The Isoelectric Point Of Lysine The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average. Determine the charge on an amino acid when it is not at the isoelectric point. If an amino acid has only one amino acid and only one carboxyl group, then the isoelectric point pi is calculated. The. What Is The Isoelectric Point Of Lysine.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master What Is The Isoelectric Point Of Lysine The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. Define zwitterion and isoelectric point. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average. In other words, the positively. What Is The Isoelectric Point Of Lysine.
From www.chegg.com
Solved Determine the isoelectric point of Lysine and What Is The Isoelectric Point Of Lysine The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. Determine the charge on an amino acid when it is not at the isoelectric point. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral. What Is The Isoelectric Point Of Lysine.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master What Is The Isoelectric Point Of Lysine Define zwitterion and isoelectric point. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. The isoelectric point (ip) is the ph at which the amino acid has an overall zero charge the isoelectric points (ip) of amino acids range. The isoelectric point, pi, is the. What Is The Isoelectric Point Of Lysine.
From chemistryguru.com.sg
Deduce Zwitterion and Isoelectric Point of Amino Acids What Is The Isoelectric Point Of Lysine Determine the charge on an amino acid when it is not at the isoelectric point. Define zwitterion and isoelectric point. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average. An aminoacid carrying no net charge is called. If an amino acid has only one amino acid. What Is The Isoelectric Point Of Lysine.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master What Is The Isoelectric Point Of Lysine The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. The isoelectric point, pi, is the ph of an. What Is The Isoelectric Point Of Lysine.
From www.numerade.com
SOLVED The structure of lysine, one of the essential amino acids, and What Is The Isoelectric Point Of Lysine An aminoacid carrying no net charge is called. Define zwitterion and isoelectric point. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average. Label amino acids as polar and nonpolar and as acidic, basic, or neutral. In other words, the positively charged groups are. The isoelectric point. What Is The Isoelectric Point Of Lysine.
From www.chegg.com
Solved Lysine has pKa values as illustrated on the diagram What Is The Isoelectric Point Of Lysine Define zwitterion and isoelectric point. If an amino acid has only one amino acid and only one carboxyl group, then the isoelectric point pi is calculated. Determine the charge on an amino acid when it is not at the isoelectric point. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which. What Is The Isoelectric Point Of Lysine.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master What Is The Isoelectric Point Of Lysine Define zwitterion and isoelectric point. An aminoacid carrying no net charge is called. The isoelectric point (ip) is the ph at which the amino acid has an overall zero charge the isoelectric points (ip) of amino acids range. Label amino acids as polar and nonpolar and as acidic, basic, or neutral. If an amino acid has only one amino acid. What Is The Isoelectric Point Of Lysine.
From www.chegg.com
Solved Determine the isoelectric point of amino acids lysine What Is The Isoelectric Point Of Lysine The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. The isoelectric point (ip) is the ph at which the amino acid has an overall zero charge the isoelectric points (ip) of amino acids range. The isoelectric point, pi, is the ph of. What Is The Isoelectric Point Of Lysine.
From www.semanticscholar.org
Figure 1 from Isoelectric point prediction from the amino acid sequence What Is The Isoelectric Point Of Lysine The isoelectric point (ip) is the ph at which the amino acid has an overall zero charge the isoelectric points (ip) of amino acids range. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. The isoelectric point, pi, is the ph of an aqueous solution. What Is The Isoelectric Point Of Lysine.
From glossary.periodni.com
Isoelectric Chemistry Dictionary & Glossary What Is The Isoelectric Point Of Lysine The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average. An aminoacid carrying no net charge is called. Define zwitterion. What Is The Isoelectric Point Of Lysine.
From www.alamy.com
Amino acid Lysine. Chemical molecular formula Lysine is an amino acid What Is The Isoelectric Point Of Lysine In other words, the positively charged groups are. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. Label amino acids as polar and nonpolar and as acidic, basic, or neutral. Determine the charge on an amino acid when it is not at the isoelectric point.. What Is The Isoelectric Point Of Lysine.
From scoop.eduncle.com
10. the pka values of lysine are 2.18, 8.95 and 10.79. the isoelectric What Is The Isoelectric Point Of Lysine Determine the charge on an amino acid when it is not at the isoelectric point. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules. What Is The Isoelectric Point Of Lysine.
From www.numerade.com
SOLVED Show the calculations for calculating the isoelectric point for What Is The Isoelectric Point Of Lysine The isoelectric point (ip) is the ph at which the amino acid has an overall zero charge the isoelectric points (ip) of amino acids range. Define zwitterion and isoelectric point. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. The isoelectric point, pi, is the. What Is The Isoelectric Point Of Lysine.
From www.researchgate.net
Representation of the charged states and isoelectric point of Lysine What Is The Isoelectric Point Of Lysine Define zwitterion and isoelectric point. In other words, the positively charged groups are. Label amino acids as polar and nonpolar and as acidic, basic, or neutral. An aminoacid carrying no net charge is called. If an amino acid has only one amino acid and only one carboxyl group, then the isoelectric point pi is calculated. The isoelectric point, pi, is. What Is The Isoelectric Point Of Lysine.
From www.chemzipper.com
to Chem What is the Isoelectric points of What Is The Isoelectric Point Of Lysine The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. In other words, the positively charged groups are. Define zwitterion and isoelectric point. The isoelectric point (ip) is the ph at which the amino acid has an overall zero charge the isoelectric points (ip) of amino. What Is The Isoelectric Point Of Lysine.
From www.researchgate.net
Representation of the charged states and isoelectric point of Lysine What Is The Isoelectric Point Of Lysine If an amino acid has only one amino acid and only one carboxyl group, then the isoelectric point pi is calculated. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average. The isoelectric point is the ph of a solution at which an amino acid or peptide. What Is The Isoelectric Point Of Lysine.
From www.youtube.com
How To Calculate The Isoelectric Point of Amino Acids and Zwitterions What Is The Isoelectric Point Of Lysine In other words, the positively charged groups are. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. The isoelectric point (ip) is the ph at which the amino acid has an overall zero charge the isoelectric points (ip) of amino acids range. Define zwitterion and. What Is The Isoelectric Point Of Lysine.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master What Is The Isoelectric Point Of Lysine Define zwitterion and isoelectric point. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average. Determine the charge on an amino acid when it is not at the isoelectric point. An aminoacid carrying no net charge is called. The isoelectric point is the ph of a solution. What Is The Isoelectric Point Of Lysine.
From scoop.eduncle.com
10. the pka values of lysine are 2.18, 8.95 and 10.79. the isoelectric What Is The Isoelectric Point Of Lysine An aminoacid carrying no net charge is called. In other words, the positively charged groups are. Determine the charge on an amino acid when it is not at the isoelectric point. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. The isoelectric point (ip) is. What Is The Isoelectric Point Of Lysine.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master What Is The Isoelectric Point Of Lysine If an amino acid has only one amino acid and only one carboxyl group, then the isoelectric point pi is calculated. In other words, the positively charged groups are. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. Determine the charge on an amino acid. What Is The Isoelectric Point Of Lysine.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master What Is The Isoelectric Point Of Lysine An aminoacid carrying no net charge is called. Define zwitterion and isoelectric point. In other words, the positively charged groups are. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid. What Is The Isoelectric Point Of Lysine.
From www.researchgate.net
Summary of different Llysine forms and charged states of silica What Is The Isoelectric Point Of Lysine Determine the charge on an amino acid when it is not at the isoelectric point. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. In other words, the positively charged groups are. The isoelectric point is the ph of a solution at. What Is The Isoelectric Point Of Lysine.
From www.doubtnut.com
The pK_(a) values of ionisable groups in lysine are 2.18, 8.95 and 10. What Is The Isoelectric Point Of Lysine The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. The isoelectric point (ip) is the ph at which the amino acid has an overall zero charge the isoelectric points (ip) of amino acids range. The isoelectric point, pi, is the ph of an aqueous solution. What Is The Isoelectric Point Of Lysine.
From study.com
Calculating Isoelectric Point Definition & Formula Video & Lesson What Is The Isoelectric Point Of Lysine The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. If an amino acid has only one amino acid. What Is The Isoelectric Point Of Lysine.
From www.chegg.com
Solved What is the isoelectric point of lysine within in What Is The Isoelectric Point Of Lysine Determine the charge on an amino acid when it is not at the isoelectric point. An aminoacid carrying no net charge is called. Label amino acids as polar and nonpolar and as acidic, basic, or neutral. The isoelectric point (ip) is the ph at which the amino acid has an overall zero charge the isoelectric points (ip) of amino acids. What Is The Isoelectric Point Of Lysine.
From www.youtube.com
Ch.3 Amino Acid part 5 Titration curve of basic amino acids (Histidine What Is The Isoelectric Point Of Lysine An aminoacid carrying no net charge is called. Label amino acids as polar and nonpolar and as acidic, basic, or neutral. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. Determine the charge on an amino acid when it is not at the isoelectric point.. What Is The Isoelectric Point Of Lysine.
From www.numerade.com
SOLVED The amino acids pictured below are shown in their unionized What Is The Isoelectric Point Of Lysine The isoelectric point (ip) is the ph at which the amino acid has an overall zero charge the isoelectric points (ip) of amino acids range. An aminoacid carrying no net charge is called. In other words, the positively charged groups are. Determine the charge on an amino acid when it is not at the isoelectric point. Label amino acids as. What Is The Isoelectric Point Of Lysine.
From scoop.eduncle.com
Based on the information given below, the isoelectric point (pI) of What Is The Isoelectric Point Of Lysine If an amino acid has only one amino acid and only one carboxyl group, then the isoelectric point pi is calculated. The isoelectric point is the ph of a solution at which an amino acid or peptide is neutral and carries zero net electric charge. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid. What Is The Isoelectric Point Of Lysine.
From www.slideserve.com
PPT Human Biochemistry PowerPoint Presentation, free download ID What Is The Isoelectric Point Of Lysine Determine the charge on an amino acid when it is not at the isoelectric point. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. Label amino acids as polar and nonpolar and as acidic, basic, or neutral. Define zwitterion and isoelectric point.. What Is The Isoelectric Point Of Lysine.
From www.chegg.com
Solved QUESTION 8 What is the isoelectric point of lysine What Is The Isoelectric Point Of Lysine In other words, the positively charged groups are. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average. The isoelectric. What Is The Isoelectric Point Of Lysine.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master What Is The Isoelectric Point Of Lysine In other words, the positively charged groups are. Determine the charge on an amino acid when it is not at the isoelectric point. If an amino acid has only one amino acid and only one carboxyl group, then the isoelectric point pi is calculated. The isoelectric point (ip) is the ph at which the amino acid has an overall zero. What Is The Isoelectric Point Of Lysine.
From www.youtube.com
Calculate Isoelectric point of Lysine Amino acid JAM 2019 Chemistry What Is The Isoelectric Point Of Lysine In other words, the positively charged groups are. Label amino acids as polar and nonpolar and as acidic, basic, or neutral. Determine the charge on an amino acid when it is not at the isoelectric point. Define zwitterion and isoelectric point. The isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which. What Is The Isoelectric Point Of Lysine.