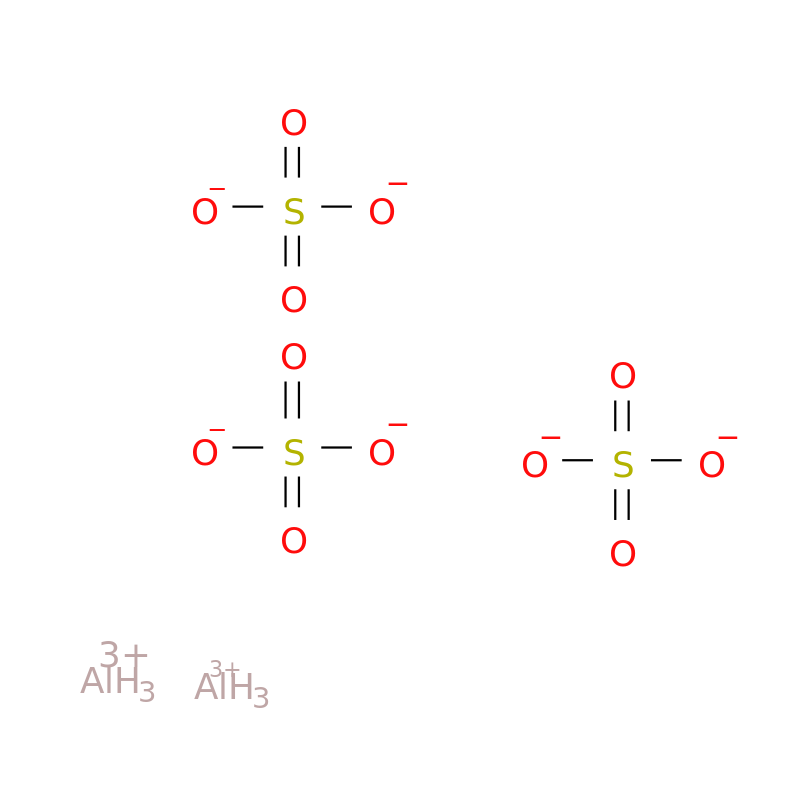
Aluminum Sulfate Molecular Formula . It is a white crystalline solid in its anhydrous. All the remaining transition metals form multiple. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Al 2 o 12 s 3. In this video we'll write the correct formula for aluminum sulfate, al (so4)3.to write the. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. The solution that results is then evaporated and allowed to.
from www.drugs.com
Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. Al 2 o 12 s 3. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. In this video we'll write the correct formula for aluminum sulfate, al (so4)3.to write the. The solution that results is then evaporated and allowed to. All the remaining transition metals form multiple. It is a white crystalline solid in its anhydrous. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations.
Aluminum sulfate brand name list from
Aluminum Sulfate Molecular Formula Al 2 o 12 s 3. The solution that results is then evaporated and allowed to. Al 2 o 12 s 3. It is a white crystalline solid in its anhydrous. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. All the remaining transition metals form multiple. In this video we'll write the correct formula for aluminum sulfate, al (so4)3.to write the. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3.
From www.fishersci.com
Aluminum Sulfate Hydrate (Crystalline/Certified ACS), Fisher Chemical Aluminum Sulfate Molecular Formula The solution that results is then evaporated and allowed to. All the remaining transition metals form multiple. Al 2 o 12 s 3. In this video we'll write the correct formula for aluminum sulfate, al (so4)3.to write the. It is a white crystalline solid in its anhydrous. Aluminum sulfate, often referred to as alum, is a chemical compound with the. Aluminum Sulfate Molecular Formula.
From www.youtube.com
How to Write the Formula for Aluminum sulfate YouTube Aluminum Sulfate Molecular Formula Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. It is a white crystalline solid in its anhydrous. In this video we'll write the correct formula for aluminum sulfate, al (so4)3.to write the. All the remaining transition metals form multiple. Al 2 o 12 s 3. The solution that results. Aluminum Sulfate Molecular Formula.
From www.dreamstime.com
Aluminium Sulfate Molecular Structure, 3d Model Molecule, E520 Aluminum Sulfate Molecular Formula Al 2 o 12 s 3. All the remaining transition metals form multiple. The solution that results is then evaporated and allowed to. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. Aluminum Sulfate Molecular Formula.
From inci.guide
Aluminum sulfate Ingredient INCIGuide Aluminum Sulfate Molecular Formula Al 2 o 12 s 3. In this video we'll write the correct formula for aluminum sulfate, al (so4)3.to write the. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. All the remaining transition metals form multiple. The solution that results is then evaporated and allowed to.. Aluminum Sulfate Molecular Formula.
From aydin-blognorton.blogspot.com
What Is the Formula for Aluminum Sulfite Aluminum Sulfate Molecular Formula All the remaining transition metals form multiple. It is a white crystalline solid in its anhydrous. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Aluminum sulfate, often referred to. Aluminum Sulfate Molecular Formula.
From www.youtube.com
How to write the formula for aluminum sulfate YouTube Aluminum Sulfate Molecular Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. In this video we'll write the correct formula for aluminum sulfate, al (so4)3.to write the. Aluminium sulphate. Aluminum Sulfate Molecular Formula.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation ID2031508 Aluminum Sulfate Molecular Formula Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. All the remaining transition metals form multiple. Al 2 o 12 s 3. It is a white crystalline solid. Aluminum Sulfate Molecular Formula.
From www.meritnation.com
Write down the formulae of aluminium sulphate and ethanol Ans Aluminum Sulfate Molecular Formula It is a white crystalline solid in its anhydrous. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. Aluminum sulfate, often referred to as alum, is. Aluminum Sulfate Molecular Formula.
From www.drugs.com
Aluminum sulfate brand name list from Aluminum Sulfate Molecular Formula It is a white crystalline solid in its anhydrous. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. The solution that results is then evaporated and allowed to. All the remaining transition metals form multiple. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose. Aluminum Sulfate Molecular Formula.
From www.dreamstime.com
Aluminium Sulfate Molecule 3d, Molecular Structure, Ball and Stick Aluminum Sulfate Molecular Formula Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. All the remaining transition metals form multiple. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where. Aluminum Sulfate Molecular Formula.
From www.youtube.com
How to write chemical formula of Aluminium sulphate Chemical formula Aluminum Sulfate Molecular Formula All the remaining transition metals form multiple. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. In this video we'll write the correct formula for aluminum. Aluminum Sulfate Molecular Formula.
From www.youtube.com
Equation for Al2(SO4)3 + H2O (Aluminum sulfate + Water) YouTube Aluminum Sulfate Molecular Formula It is a white crystalline solid in its anhydrous. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium. Aluminum Sulfate Molecular Formula.
From clairekruwmunoz.blogspot.com
Give the Correct Formula for Aluminum Sulfate. ClairekruwMunoz Aluminum Sulfate Molecular Formula Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. It is a white crystalline solid in its anhydrous. All the remaining transition metals form multiple. Aluminum. Aluminum Sulfate Molecular Formula.
From www.mumbaichemicalcompany.in
Aluminum Sulfate Non Ferric Alum AL2(SO4)3 ETP Chemical Aluminum Sulfate Molecular Formula All the remaining transition metals form multiple. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. The solution that results is then evaporated and allowed to. It is a white crystalline solid in its anhydrous. Aluminum sulfate, often referred to as alum, is a chemical compound with. Aluminum Sulfate Molecular Formula.
From cartoondealer.com
Aluminium Sulfate Molecule, Firming Agent E520, Molecular Structure Aluminum Sulfate Molecular Formula Al 2 o 12 s 3. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. In this video we'll write the correct formula for aluminum sulfate, al (so4)3.to write the. It is a white crystalline solid in its anhydrous. Aluminum sulfate, often referred to as alum, is a chemical compound with. Aluminum Sulfate Molecular Formula.
From www.dreamstime.com
Aluminium Sulfate Molecule, Structural Chemical Formula, Ballandstick Aluminum Sulfate Molecular Formula The solution that results is then evaporated and allowed to. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured. Aluminum Sulfate Molecular Formula.
From www.chegg.com
Solved Give the correct formula for aluminum sulfate. O Aluminum Sulfate Molecular Formula Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form. Aluminum Sulfate Molecular Formula.
From www.fishersci.com
Aluminium sulfate, 99.999, (trace metal basis), extra pure, ACROS Aluminum Sulfate Molecular Formula Al 2 o 12 s 3. It is a white crystalline solid in its anhydrous. All the remaining transition metals form multiple. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated. Aluminum Sulfate Molecular Formula.
From www.youtube.com
What is the chemical formula of Aluminium sulphate ? // chemical name Aluminum Sulfate Molecular Formula The solution that results is then evaporated and allowed to. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Al 2 o 12 s 3. All the remaining transition metals form multiple. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated. Aluminum Sulfate Molecular Formula.
From www.dutchchems.com
Want to buy Aluminium sulphate? Order it today at DutchChems Aluminum Sulfate Molecular Formula All the remaining transition metals form multiple. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. It is a white crystalline solid in its anhydrous. Aluminum sulfate, represented by. Aluminum Sulfate Molecular Formula.
From www.museoinclusivo.com
What is the Chemical Formula for Aluminum Sulfate? Aluminum Profile Blog Aluminum Sulfate Molecular Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. It is a white crystalline solid in its anhydrous. The solution that results is then evaporated and allowed to. In this video we'll write the correct formula for aluminum sulfate, al (so4)3.to write the. Aluminum sulfate, often referred to. Aluminum Sulfate Molecular Formula.
From www.museoinclusivo.com
Exploring Aluminum Sulfate Formula Uses, Chemical Properties, and Aluminum Sulfate Molecular Formula It is a white crystalline solid in its anhydrous. In this video we'll write the correct formula for aluminum sulfate, al (so4)3.to write the. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. All the remaining transition metals form multiple. The lighter group 3a metals (aluminum, galium and indium), along. Aluminum Sulfate Molecular Formula.
From www.youtube.com
How to Write the Formula for Sodium aluminum sulfate YouTube Aluminum Sulfate Molecular Formula All the remaining transition metals form multiple. Al 2 o 12 s 3. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. It is a white crystalline solid in its anhydrous. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium. Aluminum Sulfate Molecular Formula.
From www.alamy.com
3D image of Aluminum sulfate skeletal formula molecular chemical Aluminum Sulfate Molecular Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. All the remaining transition metals form multiple. It is a white crystalline solid in its anhydrous. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Al 2 o 12 s. Aluminum Sulfate Molecular Formula.
From www.museoinclusivo.com
Exploring Aluminum Sulfate Formula Uses, Chemical Properties, and Aluminum Sulfate Molecular Formula Al 2 o 12 s 3. It is a white crystalline solid in its anhydrous. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. The solution that results is then evaporated and allowed to. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are. Aluminum Sulfate Molecular Formula.
From www.fishersci.com
Aluminum Sulfate Hydrate, Technical, Fisher Chemical, Quantity 3 kg Aluminum Sulfate Molecular Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. In this video we'll write the correct formula for aluminum sulfate, al (so4)3.to write the. The solution that results is then evaporated and allowed to. It is a white crystalline solid in its anhydrous. Aluminum sulfate, often referred to. Aluminum Sulfate Molecular Formula.
From www.museoinclusivo.com
Molecular Mass of Aluminum Sulfate An Overview Aluminum Profile Blog Aluminum Sulfate Molecular Formula The solution that results is then evaporated and allowed to. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Al 2 o 12 s 3. All the remaining transition metals form multiple. It is a white crystalline solid in its anhydrous. Aluminum sulfate, often referred to as alum,. Aluminum Sulfate Molecular Formula.
From www.youtube.com
How to write Molecular formula of Aluminium Sulphate Chemical formula Aluminum Sulfate Molecular Formula The solution that results is then evaporated and allowed to. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. In this video we'll write the correct formula for aluminum sulfate, al (so4)3.to write the. Al 2 o 12 s 3. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured. Aluminum Sulfate Molecular Formula.
From aluminumsulfatepitsukata.blogspot.com
Aluminum Sulfate Chemical Formula For Sodium Aluminum Sulfate Aluminum Sulfate Molecular Formula Al 2 o 12 s 3. It is a white crystalline solid in its anhydrous. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. The solution that results is then evaporated and allowed to. All the remaining transition metals form multiple. The lighter group 3a metals (aluminum, galium and indium), along. Aluminum Sulfate Molecular Formula.
From aluminumsulfatepitsukata.blogspot.com
Aluminum Sulfate Chemical Formula For Sodium Aluminum Sulfate Aluminum Sulfate Molecular Formula In this video we'll write the correct formula for aluminum sulfate, al (so4)3.to write the. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Al 2 o 12 s 3. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide.. Aluminum Sulfate Molecular Formula.
From www.dreamstime.com
Aluminium Sulfate Molecular Structure 3d, Flat Model, Firming Agent Aluminum Sulfate Molecular Formula Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. In. Aluminum Sulfate Molecular Formula.
From www.pinterest.com
Aluminium sulfate Al2(SO4)3 Molecular Geometry Hybridization Aluminum Sulfate Molecular Formula All the remaining transition metals form multiple. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminum sulfate, represented by the chemical formula al₂(so₄)₃, has a structured composition where two aluminum atoms are bonded with three sulfate groups. Al 2 o 12 s 3. It is a white. Aluminum Sulfate Molecular Formula.
From hxewxvhin.blob.core.windows.net
How To Aluminum Sulfate Formula at Caitlin Hardin blog Aluminum Sulfate Molecular Formula Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. It is a white crystalline solid in its anhydrous. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. The solution that results is then evaporated and allowed to. Aluminum sulfate, represented by. Aluminum Sulfate Molecular Formula.
From molekula.com
Purchase Aluminum sulfate hexadecahydrate [16828118] online • Catalog Aluminum Sulfate Molecular Formula The solution that results is then evaporated and allowed to. All the remaining transition metals form multiple. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Al 2 o 12. Aluminum Sulfate Molecular Formula.
From www.fishersci.se
Aluminum Sulfate Hexadecahydrate, Technical, Fisher Chemical Fisher Aluminum Sulfate Molecular Formula It is a white crystalline solid in its anhydrous. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. The solution that results is then evaporated and allowed to. In this. Aluminum Sulfate Molecular Formula.