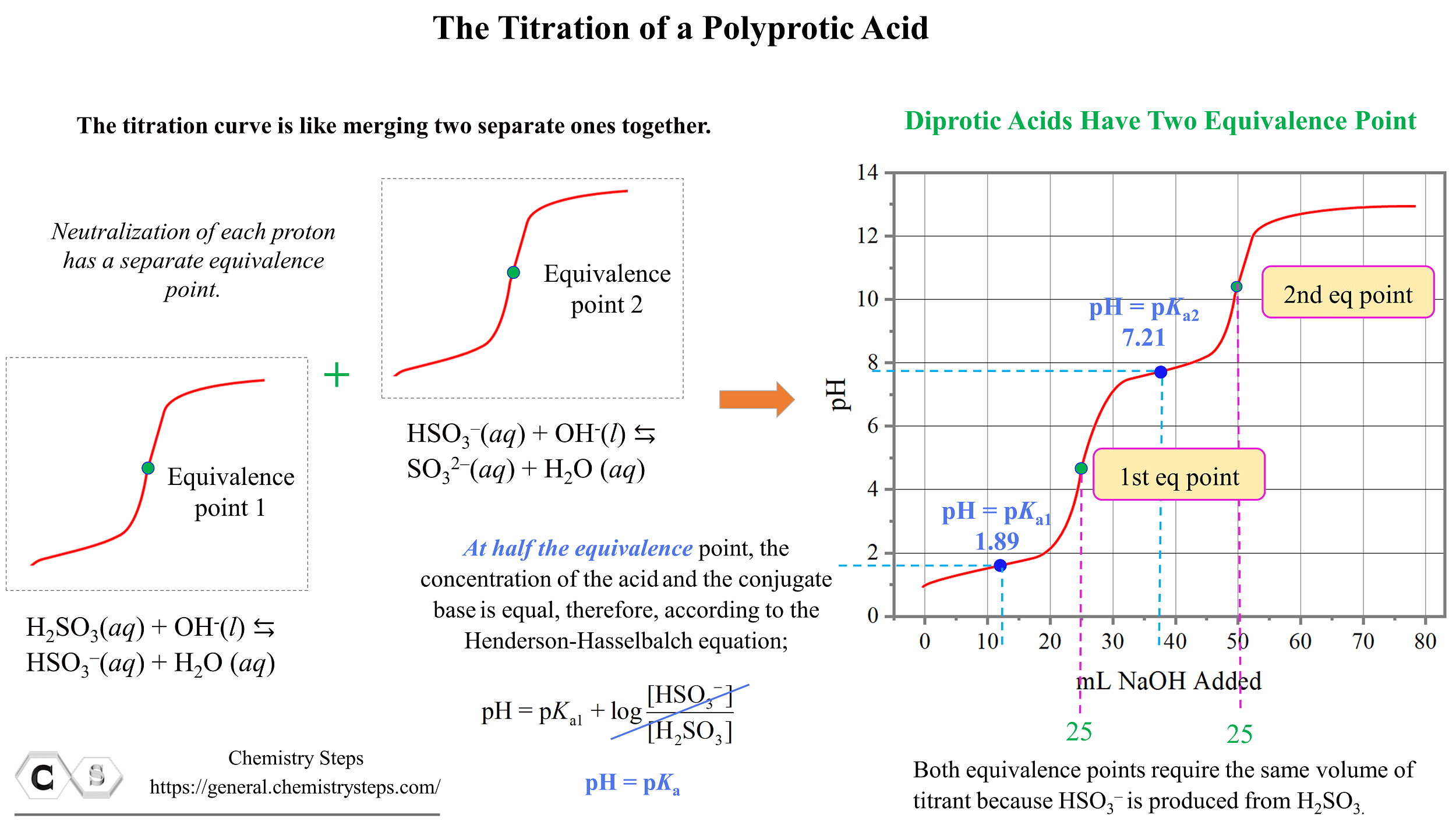
Titration Equivalence Point . Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Learn what equivalence point is in chemistry and how to determine it in different types of titrations. The equivalence point of a titration. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sorting out some confusing terms. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is.
from
Learn what equivalence point is in chemistry and how to determine it in different types of titrations. Sorting out some confusing terms. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow.
Titration Equivalence Point The equivalence point of a titration. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence point of a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Sorting out some confusing terms. Learn what equivalence point is in chemistry and how to determine it in different types of titrations. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration Equivalence Point When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. At the equivalence point in a neutralization, the moles. Titration Equivalence Point.
From www.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Chemistry JoVE Titration Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Sorting out some confusing terms. Learn what equivalence point is in chemistry and how to determine. Titration Equivalence Point.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. At the equivalence point in a neutralization, the moles. Titration Equivalence Point.
From
Titration Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. A titration is a volumetric technique in which a. Titration Equivalence Point.
From
Titration Equivalence Point Sorting out some confusing terms. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Learn what equivalence point is in chemistry and how to determine it in different types of titrations. When 25 ml of titrant has been added (the equivalence point), the. Titration Equivalence Point.
From
Titration Equivalence Point Learn what equivalence point is in chemistry and how to determine it in different types of titrations. The equivalence point of a titration. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Sketch out a plot representing the titration of a weak monoprotic acid. Titration Equivalence Point.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration Equivalence Point Learn what equivalence point is in chemistry and how to determine it in different types of titrations. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The equivalence point of a titration. At the equivalence point in a. Titration Equivalence Point.
From
Titration Equivalence Point The equivalence point of a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Sorting out some confusing terms. Learn what equivalence point is in chemistry and how to determine it in different types of titrations. When 25 ml of titrant has. Titration Equivalence Point.
From slideplayer.com
AP Chemistry Lunch Review ppt download Titration Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sorting out some confusing terms. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Sketch out a plot representing the titration of a weak monoprotic acid. Titration Equivalence Point.
From mungfali.com
Acid Base Titration Graph Titration Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The equivalence point. Titration Equivalence Point.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point Titration Equivalence Point The equivalence point of a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Sorting out some confusing terms. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Learn what equivalence point is. Titration Equivalence Point.
From ar.inspiredpencil.com
H3po4 Titration Curve Titration Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a titration. Learn what equivalence point is in chemistry and how to determine it in different types of titrations. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit. Titration Equivalence Point.
From
Titration Equivalence Point When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Learn what equivalence point is in chemistry and how to determine it in different types of titrations. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base.. Titration Equivalence Point.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Equivalence Point Sorting out some confusing terms. Learn what equivalence point is in chemistry and how to determine it in different types of titrations. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. At the equivalence point in a neutralization, the moles of acid are equal. Titration Equivalence Point.
From
Titration Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The equivalence point of a titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. When 25 ml of titrant has been added (the equivalence. Titration Equivalence Point.
From
Titration Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. When 25 ml of titrant has been added (the equivalence point), the ph is well above. Titration Equivalence Point.
From
Titration Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Sorting out some confusing terms. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Titration Equivalence Point.
From
Titration Equivalence Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Sorting out some confusing terms. The equivalence point of a titration. Learn what equivalence point is in chemistry and how to determine it in different types of titrations. When. Titration Equivalence Point.
From mungfali.com
Acid Base Titration Graph Titration Equivalence Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a titration. Sorting out some confusing terms.. Titration Equivalence Point.
From
Titration Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sorting out some confusing terms. Learn what equivalence point is in chemistry and how to determine it in different types of titrations. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and. Titration Equivalence Point.
From
Titration Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. When 25 ml of titrant has been added (the equivalence point), the ph is well above. Titration Equivalence Point.
From
Titration Equivalence Point Sorting out some confusing terms. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Learn what equivalence point is in chemistry and how to determine it in different types of titrations. Sketch out a plot representing the titration. Titration Equivalence Point.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Equivalence Point Learn what equivalence point is in chemistry and how to determine it in different types of titrations. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The equivalence point of a titration. Sorting out some confusing terms. At the equivalence point in a. Titration Equivalence Point.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Titration Equivalence Point Learn what equivalence point is in chemistry and how to determine it in different types of titrations. The equivalence point of a titration. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. A titration is a volumetric technique in which a solution of one. Titration Equivalence Point.
From www.aiophotoz.com
How To Find The Half Equivalence Point In A Titration Graph Sciencing Titration Equivalence Point Sorting out some confusing terms. The equivalence point of a titration. Learn what equivalence point is in chemistry and how to determine it in different types of titrations. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. A titration is a volumetric technique in. Titration Equivalence Point.
From
Titration Equivalence Point When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence point of a titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is a volumetric technique in which a solution of. Titration Equivalence Point.
From
Titration Equivalence Point When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. At the equivalence point in a neutralization, the moles. Titration Equivalence Point.
From mavink.com
Titration Labeled Titration Equivalence Point Learn what equivalence point is in chemistry and how to determine it in different types of titrations. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow.. Titration Equivalence Point.
From saylordotorg.github.io
AcidBase Titrations Titration Equivalence Point Sorting out some confusing terms. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Titration Equivalence Point.
From www.slideserve.com
PPT Acidity Constant by pH Titration Curves PowerPoint Presentation Titration Equivalence Point At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added. Titration Equivalence Point.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Equivalence Point Sorting out some confusing terms. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The equivalence point of a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of. Titration Equivalence Point.
From
Titration Equivalence Point Learn what equivalence point is in chemistry and how to determine it in different types of titrations. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Titration Equivalence Point.
From
Titration Equivalence Point The equivalence point of a titration. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Learn what equivalence point is in chemistry and how to determine it in different types of titrations. Sketch out a plot representing the titration of a weak monoprotic acid. Titration Equivalence Point.
From
Titration Equivalence Point The equivalence point of a titration. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Sorting out some confusing terms. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. Titration Equivalence Point.
From
Titration Equivalence Point Sorting out some confusing terms. Learn what equivalence point is in chemistry and how to determine it in different types of titrations. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. At the equivalence point in a neutralization, the moles of acid are equal. Titration Equivalence Point.