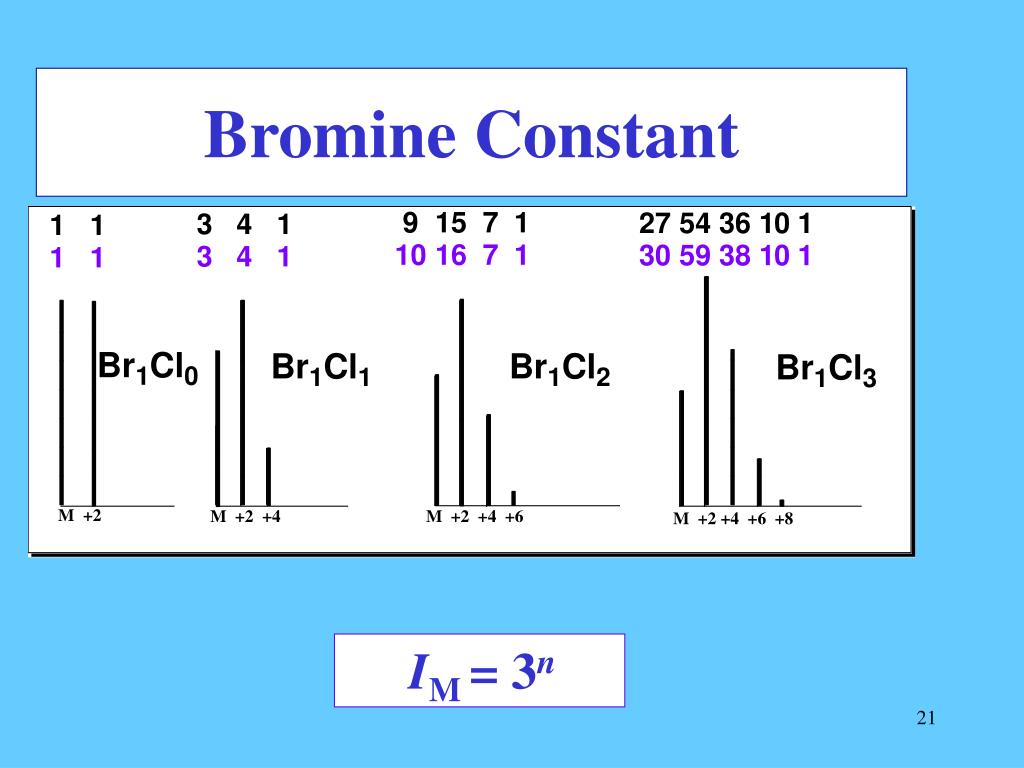
Bromine Isotope Pattern . These two peaks correspond to the molecular. Br isotope effects are crucial in describing brominated organics fate in the environment. Compare your measured data with the theoretical pattern,. That means that a compound containing 1 bromine atom will have two peaks in. Isotope symbol with mass number (superscript; Number of nucleons) and atomic number (subscript;. 49.5 if you want to be fussy!). Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : The patterns are highlighted in the green boxes:. The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have the 1:1 relative abundance characteristic of bromine. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments.
from www.slideserve.com
The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have the 1:1 relative abundance characteristic of bromine. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : Br isotope effects are crucial in describing brominated organics fate in the environment. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. These two peaks correspond to the molecular. 49.5 if you want to be fussy!). Isotope symbol with mass number (superscript; Number of nucleons) and atomic number (subscript;. The patterns are highlighted in the green boxes:. That means that a compound containing 1 bromine atom will have two peaks in.
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds
Bromine Isotope Pattern Isotope symbol with mass number (superscript; This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. These two peaks correspond to the molecular. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have the 1:1 relative abundance characteristic of bromine. Br isotope effects are crucial in describing brominated organics fate in the environment. 49.5 if you want to be fussy!). The patterns are highlighted in the green boxes:. Isotope symbol with mass number (superscript; Compare your measured data with the theoretical pattern,. That means that a compound containing 1 bromine atom will have two peaks in. Number of nucleons) and atomic number (subscript;.
From www.slideserve.com
PPT Bromine PowerPoint Presentation ID2319111 Bromine Isotope Pattern Br isotope effects are crucial in describing brominated organics fate in the environment. Isotope symbol with mass number (superscript; This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have the 1:1 relative. Bromine Isotope Pattern.
From blog.sepscience.com
The Role of Isotope Peak Intensities Obtained Using Mass Spectrometry Bromine Isotope Pattern Number of nucleons) and atomic number (subscript;. Isotope symbol with mass number (superscript; 49.5 if you want to be fussy!). These two peaks correspond to the molecular. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : Br isotope effects are crucial in describing brominated organics fate in the environment. The bromine isotope pattern. Bromine Isotope Pattern.
From mavink.com
Lewis Structure For Bromine Bromine Isotope Pattern Isotope symbol with mass number (superscript; This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. 49.5 if you want to be fussy!). The patterns are highlighted in the green boxes:. Compare your measured data with the theoretical pattern,. These two peaks correspond to the molecular. Br isotope effects. Bromine Isotope Pattern.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms Bromine Isotope Pattern That means that a compound containing 1 bromine atom will have two peaks in. The patterns are highlighted in the green boxes:. The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have the 1:1 relative abundance characteristic of bromine. Isotope symbol with mass number (superscript; These two peaks correspond to the molecular. Compare. Bromine Isotope Pattern.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Bromine Isotope Pattern Number of nucleons) and atomic number (subscript;. These two peaks correspond to the molecular. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. The bromine isotope pattern is seen in the peaks. Bromine Isotope Pattern.
From www.youtube.com
Notation for Isotopes of Bromine (Br) YouTube Bromine Isotope Pattern That means that a compound containing 1 bromine atom will have two peaks in. These two peaks correspond to the molecular. The patterns are highlighted in the green boxes:. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Br isotope effects are crucial in describing brominated organics fate. Bromine Isotope Pattern.
From www.slideserve.com
PPT Analysis of the Isotope Patterns of Organic Sulfur Containing Bromine Isotope Pattern This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : The patterns are highlighted in the green boxes:. These two peaks correspond to the molecular. Compare your measured data with the theoretical pattern,.. Bromine Isotope Pattern.
From www.slideserve.com
PPT Factoring Isotope Patterns Chlorine and Bromine with nitrogen Bromine Isotope Pattern That means that a compound containing 1 bromine atom will have two peaks in. These two peaks correspond to the molecular. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : Br isotope effects are crucial in describing brominated organics fate in the environment. This table shows information about naturally occuring isotopes, their atomic. Bromine Isotope Pattern.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Bromine Isotope Pattern This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : Isotope symbol with mass number (superscript; The patterns are highlighted in the green boxes:. The bromine isotope pattern is seen in the peaks. Bromine Isotope Pattern.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Bromine Isotope Pattern These two peaks correspond to the molecular. 49.5 if you want to be fussy!). Compare your measured data with the theoretical pattern,. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have. Bromine Isotope Pattern.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Bromine Isotope Pattern Br isotope effects are crucial in describing brominated organics fate in the environment. Compare your measured data with the theoretical pattern,. Number of nucleons) and atomic number (subscript;. That means that a compound containing 1 bromine atom will have two peaks in. The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have the. Bromine Isotope Pattern.
From www.slideshare.net
Bromine berry Bromine Isotope Pattern The patterns are highlighted in the green boxes:. 49.5 if you want to be fussy!). This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have the 1:1 relative abundance characteristic of bromine.. Bromine Isotope Pattern.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms Bromine Isotope Pattern Isotope symbol with mass number (superscript; This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : These two peaks correspond to the molecular. Number of nucleons) and atomic number (subscript;. The bromine isotope. Bromine Isotope Pattern.
From www.sciencephoto.com
Bromine, atomic structure Stock Image C018/3716 Science Photo Library Bromine Isotope Pattern That means that a compound containing 1 bromine atom will have two peaks in. 49.5 if you want to be fussy!). Number of nucleons) and atomic number (subscript;. These two peaks correspond to the molecular. The patterns are highlighted in the green boxes:. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : This. Bromine Isotope Pattern.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Bromine Isotope Pattern The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have the 1:1 relative abundance characteristic of bromine. These two peaks correspond to the molecular. Isotope symbol with mass number (superscript; The patterns are highlighted in the green boxes:. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5. Bromine Isotope Pattern.
From slideplayer.com
Advanced Pharmaceutical Analysis Mass spectrometry ppt download Bromine Isotope Pattern This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Compare your measured data with the theoretical pattern,. Isotope symbol with mass number (superscript; 49.5 if you want to be fussy!). The patterns are highlighted in the green boxes:. Number of nucleons) and atomic number (subscript;. Bromine has two. Bromine Isotope Pattern.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation ID2894903 Bromine Isotope Pattern Number of nucleons) and atomic number (subscript;. That means that a compound containing 1 bromine atom will have two peaks in. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. 49.5 if you want to be fussy!). Br isotope effects are crucial in describing brominated organics fate in. Bromine Isotope Pattern.
From www.slideserve.com
PPT Factoring Isotope Patterns Chlorine and Bromine with nitrogen Bromine Isotope Pattern These two peaks correspond to the molecular. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : 49.5 if you want to be fussy!). This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Br isotope effects are crucial in describing brominated organics. Bromine Isotope Pattern.
From www.researchgate.net
Comparison of mass values and isotopic patterns 1) experimental m/z Bromine Isotope Pattern Number of nucleons) and atomic number (subscript;. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. These two peaks correspond to the molecular. The bromine isotope pattern is seen in the peaks. Bromine Isotope Pattern.
From www.numerade.com
SOLVEDNaturally occurring bromine consists of two isotopes, ^79 Br and Bromine Isotope Pattern Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : Number of nucleons) and atomic number (subscript;. The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have the 1:1 relative abundance characteristic of bromine. That means that a compound containing 1 bromine atom will have two peaks. Bromine Isotope Pattern.
From www.researchgate.net
Chlorine/bromine isotope ratios (IR 37 Cl/ 35 Cl or 81 Br/ 79 Br) of Bromine Isotope Pattern This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. That means that a compound containing 1 bromine atom will have two peaks in. The patterns are highlighted in the green boxes:. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : 49.5. Bromine Isotope Pattern.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Bromine Isotope Pattern The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have the 1:1 relative abundance characteristic of bromine. Br isotope effects are crucial in describing brominated organics fate in the environment. That means that a compound containing 1 bromine atom will have two peaks in. Compare your measured data with the theoretical pattern,. This. Bromine Isotope Pattern.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Bromine Isotope Pattern Br isotope effects are crucial in describing brominated organics fate in the environment. Isotope symbol with mass number (superscript; Compare your measured data with the theoretical pattern,. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have. Bromine Isotope Pattern.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Bromine Isotope Pattern Isotope symbol with mass number (superscript; Br isotope effects are crucial in describing brominated organics fate in the environment. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. The patterns are highlighted in the green boxes:. 49.5 if you want to be fussy!). Number of nucleons) and atomic. Bromine Isotope Pattern.
From www.slideserve.com
PPT Factoring Isotope Patterns Chlorine and Bromine with nitrogen Bromine Isotope Pattern The patterns are highlighted in the green boxes:. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Br isotope effects are crucial in describing brominated organics fate in the environment. Number of nucleons) and atomic number (subscript;. Bromine has two isotopes, 79 br and 81 br in an. Bromine Isotope Pattern.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Isotope Pattern Number of nucleons) and atomic number (subscript;. The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have the 1:1 relative abundance characteristic of bromine. The patterns are highlighted in the green boxes:. Compare your measured data with the theoretical pattern,. Isotope symbol with mass number (superscript; That means that a compound containing 1. Bromine Isotope Pattern.
From www.researchgate.net
Relative variations of chlorine/bromine and carbon isotope ratios (Δ 37 Bromine Isotope Pattern That means that a compound containing 1 bromine atom will have two peaks in. Bromine has two isotopes, 79 br and 81 br in an approximately 1:1 ratio (50.5 : Number of nucleons) and atomic number (subscript;. Compare your measured data with the theoretical pattern,. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their. Bromine Isotope Pattern.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Isotope Pattern Compare your measured data with the theoretical pattern,. Isotope symbol with mass number (superscript; 49.5 if you want to be fussy!). The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have the 1:1 relative abundance characteristic of bromine. That means that a compound containing 1 bromine atom will have two peaks in. This. Bromine Isotope Pattern.
From www.webelements.com
Elements Periodic Table » Bromine » properties of free atoms Bromine Isotope Pattern Isotope symbol with mass number (superscript; This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. These two peaks correspond to the molecular. Number of nucleons) and atomic number (subscript;. Br isotope effects are crucial in describing brominated organics fate in the environment. That means that a compound containing. Bromine Isotope Pattern.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Bromine Isotope Pattern Number of nucleons) and atomic number (subscript;. Isotope symbol with mass number (superscript; This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Compare your measured data with the theoretical pattern,. Br isotope effects are crucial in describing brominated organics fate in the environment. 49.5 if you want to. Bromine Isotope Pattern.
From www.slideserve.com
PPT Factoring Isotope Patterns Chlorine and Bromine with nitrogen Bromine Isotope Pattern Compare your measured data with the theoretical pattern,. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. These two peaks correspond to the molecular. That means that a compound containing 1 bromine atom will have two peaks in. Number of nucleons) and atomic number (subscript;. The patterns are. Bromine Isotope Pattern.
From nap.nationalacademies.org
Table of Isotopes of Fluorine, Chlorine, Bromine and Iodine The Bromine Isotope Pattern The bromine isotope pattern is seen in the peaks at 156 m/z and 158 m/z which have the 1:1 relative abundance characteristic of bromine. Number of nucleons) and atomic number (subscript;. Compare your measured data with the theoretical pattern,. That means that a compound containing 1 bromine atom will have two peaks in. These two peaks correspond to the molecular.. Bromine Isotope Pattern.
From www.dreamstime.com
Element of Bromine stock vector. Illustration of college 104400483 Bromine Isotope Pattern Compare your measured data with the theoretical pattern,. The patterns are highlighted in the green boxes:. Number of nucleons) and atomic number (subscript;. Isotope symbol with mass number (superscript; That means that a compound containing 1 bromine atom will have two peaks in. These two peaks correspond to the molecular. 49.5 if you want to be fussy!). Bromine has two. Bromine Isotope Pattern.
From www.slideserve.com
PPT Factoring Isotope Patterns Chlorine and Bromine with nitrogen Bromine Isotope Pattern Br isotope effects are crucial in describing brominated organics fate in the environment. 49.5 if you want to be fussy!). That means that a compound containing 1 bromine atom will have two peaks in. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Bromine has two isotopes, 79. Bromine Isotope Pattern.
From www.istockphoto.com
Bromine Isotopes Stock Illustration Download Image Now Isotope Bromine Isotope Pattern Compare your measured data with the theoretical pattern,. Isotope symbol with mass number (superscript; These two peaks correspond to the molecular. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. That means that a compound containing 1 bromine atom will have two peaks in. Number of nucleons) and. Bromine Isotope Pattern.