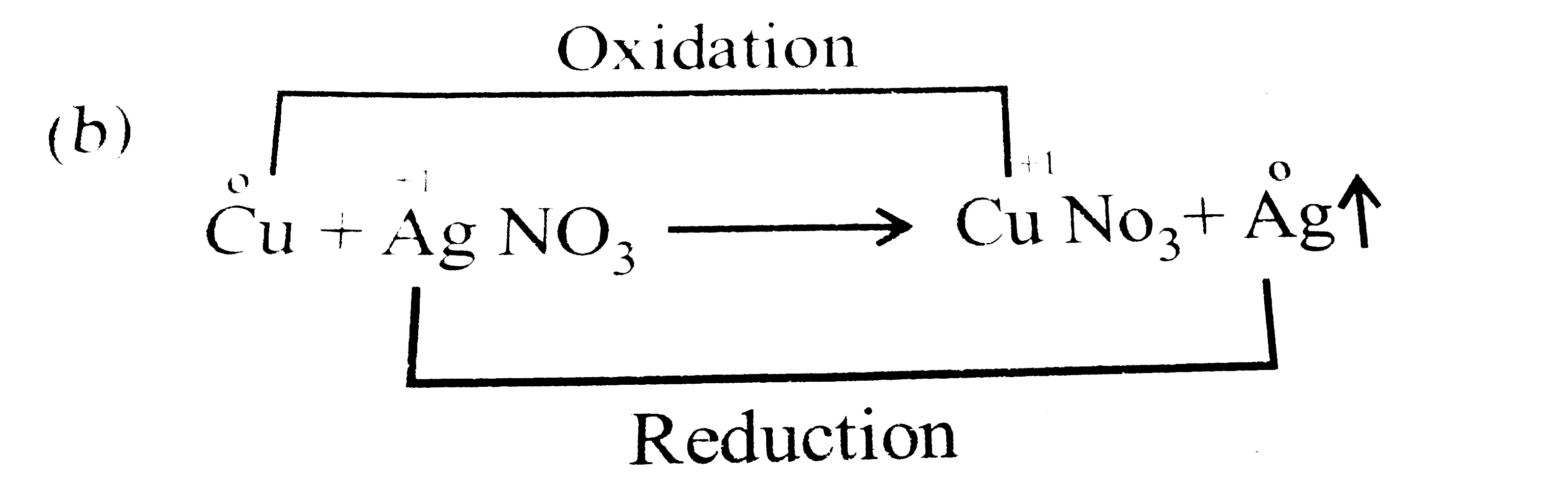
When A Copper Wire Is Placed In Silver Nitrate . Copper nitrate is seen turning the. Copper displaces silver from silver nitrate to form silver metal. Metallic copper is placed in a solution of silver nitrate. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. We will use electrochemical series to solve the. (i) when copper wire is placed in silver nitrate solution then the solution becomes blue colored. Within minutes, the wire begins to look fuzzy or furry as small silver. Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure \(\pageindex{1}\)). A single displacement reaction occurs also known as a redox reaction. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. As soon as the copper metal is added, silver metal begins to. When copper is dipped in the solution of silver nitrate, the solution turns blue. When copper wire is placed in silver nitrate solution; Silver metal forms at the surface of the copper metal.
from www.doubtnut.com
The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Copper displaces silver from silver nitrate to form silver metal. Metallic copper is placed in a solution of silver nitrate. Within minutes, the wire begins to look fuzzy or furry as small silver. As soon as the copper metal is added, silver metal begins to. (i) when copper wire is placed in silver nitrate solution then the solution becomes blue colored. We will use electrochemical series to solve the. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. When copper wire is placed in silver nitrate solution; A single displacement reaction occurs also known as a redox reaction.
When a copper rod is dipped in aqueous silver nitrate solution , the c
When A Copper Wire Is Placed In Silver Nitrate Silver metal forms at the surface of the copper metal. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure \(\pageindex{1}\)). Metallic copper is placed in a solution of silver nitrate. Within minutes, the wire begins to look fuzzy or furry as small silver. (i) when copper wire is placed in silver nitrate solution then the solution becomes blue colored. We will use electrochemical series to solve the. Silver metal forms at the surface of the copper metal. Copper nitrate is seen turning the. As soon as the copper metal is added, silver metal begins to. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Copper displaces silver from silver nitrate to form silver metal. A single displacement reaction occurs also known as a redox reaction. When copper is dipped in the solution of silver nitrate, the solution turns blue. When copper wire is placed in silver nitrate solution;
From studylib.net
CopperSilver Nitrate Reaction When A Copper Wire Is Placed In Silver Nitrate A single displacement reaction occurs also known as a redox reaction. (i) when copper wire is placed in silver nitrate solution then the solution becomes blue colored. As soon as the copper metal is added, silver metal begins to. Metallic copper is placed in a solution of silver nitrate. Copper displaces silver from silver nitrate to form silver metal. When. When A Copper Wire Is Placed In Silver Nitrate.
From exopnayqj.blob.core.windows.net
Silver Nitrate Copper Wire Balanced Equation at Maria Samuels blog When A Copper Wire Is Placed In Silver Nitrate A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. We will use electrochemical series to solve the. (i) when copper wire is placed in silver nitrate solution then the solution becomes blue colored. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver. When A Copper Wire Is Placed In Silver Nitrate.
From www.sciencephoto.com
Copper in Silver Nitrate Stock Image C002/7942 Science Photo Library When A Copper Wire Is Placed In Silver Nitrate Within minutes, the wire begins to look fuzzy or furry as small silver. When copper wire is placed in silver nitrate solution; A single displacement reaction occurs also known as a redox reaction. When copper is dipped in the solution of silver nitrate, the solution turns blue. Copper displaces silver from silver nitrate to form silver metal. A simple demonstration. When A Copper Wire Is Placed In Silver Nitrate.
From fphoto.photoshelter.com
displacement copper silver nitrate chemistry Fundamental Photographs When A Copper Wire Is Placed In Silver Nitrate Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure \(\pageindex{1}\)). When copper is dipped in the solution of silver nitrate, the solution turns blue. A single displacement reaction occurs also known as a redox reaction. When copper wire is placed in silver nitrate solution; As soon as the copper metal. When A Copper Wire Is Placed In Silver Nitrate.
From www.youtube.com
Reaction Between Copper and Silver Nitrate YouTube When A Copper Wire Is Placed In Silver Nitrate A single displacement reaction occurs also known as a redox reaction. Silver metal forms at the surface of the copper metal. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Copper nitrate is seen turning the. We will use electrochemical series to solve the. Metallic copper is placed. When A Copper Wire Is Placed In Silver Nitrate.
From www.toppr.com
Vidisha placed a copper wire in silver nitrate solution as shown in the When A Copper Wire Is Placed In Silver Nitrate When copper wire is placed in silver nitrate solution; Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure \(\pageindex{1}\)). A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. (i) when copper wire is placed in silver nitrate solution then. When A Copper Wire Is Placed In Silver Nitrate.
From www.youtube.com
Demonstration Displacement Reaction of Silver Nitrate and Copper Metal When A Copper Wire Is Placed In Silver Nitrate Metallic copper is placed in a solution of silver nitrate. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. When copper wire is placed in silver nitrate solution; A single displacement reaction occurs also known as a redox reaction. (i) when copper wire is placed in silver nitrate solution. When A Copper Wire Is Placed In Silver Nitrate.
From slideplayer.com
Introduction to Redox Mrs. Kay Chemistry 12 Chapter 18 Pages ppt download When A Copper Wire Is Placed In Silver Nitrate We will use electrochemical series to solve the. Copper displaces silver from silver nitrate to form silver metal. Silver metal forms at the surface of the copper metal. Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure \(\pageindex{1}\)). When copper wire is placed in silver nitrate solution; The reaction of. When A Copper Wire Is Placed In Silver Nitrate.
From www.doubtnut.com
When a copper rod is dipped in aqueous silver nitrate solution , the c When A Copper Wire Is Placed In Silver Nitrate Silver metal forms at the surface of the copper metal. As soon as the copper metal is added, silver metal begins to. When copper is dipped in the solution of silver nitrate, the solution turns blue. Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure \(\pageindex{1}\)). (i) when copper wire. When A Copper Wire Is Placed In Silver Nitrate.
From shaunmwilliams.com
Lecture 5 Presentation When A Copper Wire Is Placed In Silver Nitrate Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure \(\pageindex{1}\)). Within minutes, the wire begins to look fuzzy or furry as small silver. (i) when copper wire is placed in silver nitrate solution then the solution becomes blue colored. A simple demonstration of a redox reaction involves placing a solid. When A Copper Wire Is Placed In Silver Nitrate.
From sciencephoto.com
Copper in Silver Nitrate Stock Image C002/7943 Science Photo Library When A Copper Wire Is Placed In Silver Nitrate Within minutes, the wire begins to look fuzzy or furry as small silver. When copper wire is placed in silver nitrate solution; When copper is dipped in the solution of silver nitrate, the solution turns blue. We will use electrochemical series to solve the. Copper displaces silver from silver nitrate to form silver metal. As soon as the copper metal. When A Copper Wire Is Placed In Silver Nitrate.
From www.reddit.com
Single replacement reaction Copper in a Silver nitrate solution. r When A Copper Wire Is Placed In Silver Nitrate When copper is dipped in the solution of silver nitrate, the solution turns blue. When copper wire is placed in silver nitrate solution; Copper nitrate is seen turning the. Silver metal forms at the surface of the copper metal. A single displacement reaction occurs also known as a redox reaction. The reaction of copper wire with silver nitrate in aqueous. When A Copper Wire Is Placed In Silver Nitrate.
From highplainsobserver.com
GHS Chemistry Class Studies Reactions and Molar Relationships When A Copper Wire Is Placed In Silver Nitrate The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Copper displaces silver from silver nitrate to form silver metal. Metallic copper is placed in a solution of silver nitrate. A single displacement reaction occurs also known as a redox reaction. We will use electrochemical series to solve the.. When A Copper Wire Is Placed In Silver Nitrate.
From www.numerade.com
SOLVED A piece of copper wire is placed into solution of silver When A Copper Wire Is Placed In Silver Nitrate We will use electrochemical series to solve the. Metallic copper is placed in a solution of silver nitrate. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. When copper wire is placed in silver nitrate solution; As soon as the copper metal is added, silver metal begins to. The. When A Copper Wire Is Placed In Silver Nitrate.
From www.sciencephoto.com
Silver nitratecopper displacement reaction Stock Image C002/7894 When A Copper Wire Is Placed In Silver Nitrate As soon as the copper metal is added, silver metal begins to. (i) when copper wire is placed in silver nitrate solution then the solution becomes blue colored. Copper nitrate is seen turning the. Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure \(\pageindex{1}\)). We will use electrochemical series to. When A Copper Wire Is Placed In Silver Nitrate.
From brainly.in
When copper turnings are added to Silver Nitrate solution a blue When A Copper Wire Is Placed In Silver Nitrate Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure \(\pageindex{1}\)). The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. A single displacement reaction occurs also known as a redox reaction. Copper nitrate is seen turning the. Silver metal. When A Copper Wire Is Placed In Silver Nitrate.
From exopnayqj.blob.core.windows.net
Silver Nitrate Copper Wire Balanced Equation at Maria Samuels blog When A Copper Wire Is Placed In Silver Nitrate When copper wire is placed in silver nitrate solution; Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure \(\pageindex{1}\)). Copper nitrate is seen turning the. As soon as the copper metal is added, silver metal begins to. We will use electrochemical series to solve the. A single displacement reaction occurs. When A Copper Wire Is Placed In Silver Nitrate.
From www.reddit.com
Copper wire & silver nitrate solution r/VisualChemistry When A Copper Wire Is Placed In Silver Nitrate We will use electrochemical series to solve the. Metallic copper is placed in a solution of silver nitrate. Silver metal forms at the surface of the copper metal. Within minutes, the wire begins to look fuzzy or furry as small silver. Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure. When A Copper Wire Is Placed In Silver Nitrate.
From express.adobe.com
Copper and Silver Nitrate When A Copper Wire Is Placed In Silver Nitrate (i) when copper wire is placed in silver nitrate solution then the solution becomes blue colored. When copper is dipped in the solution of silver nitrate, the solution turns blue. We will use electrochemical series to solve the. Within minutes, the wire begins to look fuzzy or furry as small silver. Consider what happens when a clean piece of copper. When A Copper Wire Is Placed In Silver Nitrate.
From fyohrrkto.blob.core.windows.net
Copper Wire And Silver Nitrate Experiment at Janie Parker blog When A Copper Wire Is Placed In Silver Nitrate The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. As soon as the copper metal is added, silver metal begins to. Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure \(\pageindex{1}\)). When copper is dipped in the solution. When A Copper Wire Is Placed In Silver Nitrate.
From www.flickr.com
copper and silver nitrate lab 005 David Lieberman Flickr When A Copper Wire Is Placed In Silver Nitrate The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Within minutes, the wire begins to look fuzzy or furry as small silver. Silver metal forms at the surface of the copper metal. Consider what happens when a clean piece of copper metal is placed in a solution of. When A Copper Wire Is Placed In Silver Nitrate.
From brainly.com
Copper wire reacts with silver nitrate to form silver and copper (1 When A Copper Wire Is Placed In Silver Nitrate (i) when copper wire is placed in silver nitrate solution then the solution becomes blue colored. When copper wire is placed in silver nitrate solution; Copper nitrate is seen turning the. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Within minutes, the wire begins to look fuzzy. When A Copper Wire Is Placed In Silver Nitrate.
From www.sciencephoto.com
Copper Reacts with Silver Nitrate, 4 of 6 Stock Image C028/1082 When A Copper Wire Is Placed In Silver Nitrate We will use electrochemical series to solve the. Copper nitrate is seen turning the. Silver metal forms at the surface of the copper metal. Within minutes, the wire begins to look fuzzy or furry as small silver. Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure \(\pageindex{1}\)). When copper is. When A Copper Wire Is Placed In Silver Nitrate.
From www.coursehero.com
[Solved] When copper is placed in silver nitrate, a single displacement When A Copper Wire Is Placed In Silver Nitrate Within minutes, the wire begins to look fuzzy or furry as small silver. We will use electrochemical series to solve the. Copper nitrate is seen turning the. Copper displaces silver from silver nitrate to form silver metal. Silver metal forms at the surface of the copper metal. Consider what happens when a clean piece of copper metal is placed in. When A Copper Wire Is Placed In Silver Nitrate.
From exopnayqj.blob.core.windows.net
Silver Nitrate Copper Wire Balanced Equation at Maria Samuels blog When A Copper Wire Is Placed In Silver Nitrate As soon as the copper metal is added, silver metal begins to. We will use electrochemical series to solve the. Copper displaces silver from silver nitrate to form silver metal. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Copper nitrate is seen turning the. Metallic copper is. When A Copper Wire Is Placed In Silver Nitrate.
From www.alamy.com
Reaction of a rod of copper in a solution of silver nitrate.rs Stock When A Copper Wire Is Placed In Silver Nitrate Copper displaces silver from silver nitrate to form silver metal. Silver metal forms at the surface of the copper metal. We will use electrochemical series to solve the. When copper wire is placed in silver nitrate solution; When copper is dipped in the solution of silver nitrate, the solution turns blue. Metallic copper is placed in a solution of silver. When A Copper Wire Is Placed In Silver Nitrate.
From www.youtube.com
CBSE Class 10 Chemistry Copper reacts with Silver Nitrate Solution When A Copper Wire Is Placed In Silver Nitrate Silver metal forms at the surface of the copper metal. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. Metallic copper is placed in a solution of silver nitrate. Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure \(\pageindex{1}\)).. When A Copper Wire Is Placed In Silver Nitrate.
From uwaterloo.ca
Copper wire in a silver nitrate solution Chem 13 News Magazine When A Copper Wire Is Placed In Silver Nitrate A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. (i) when copper wire is placed in silver nitrate solution then the solution becomes blue colored. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Consider what happens. When A Copper Wire Is Placed In Silver Nitrate.
From www.sciencephoto.com
Copper in Silver Nitrate Stock Image C002/7941 Science Photo Library When A Copper Wire Is Placed In Silver Nitrate When copper is dipped in the solution of silver nitrate, the solution turns blue. When copper wire is placed in silver nitrate solution; Within minutes, the wire begins to look fuzzy or furry as small silver. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Silver metal forms. When A Copper Wire Is Placed In Silver Nitrate.
From www.numerade.com
SOLVEDChallenge When copper wire is placed into a silver nitrate When A Copper Wire Is Placed In Silver Nitrate A single displacement reaction occurs also known as a redox reaction. (i) when copper wire is placed in silver nitrate solution then the solution becomes blue colored. Metallic copper is placed in a solution of silver nitrate. Copper displaces silver from silver nitrate to form silver metal. A simple demonstration of a redox reaction involves placing a solid piece of. When A Copper Wire Is Placed In Silver Nitrate.
From fphoto.photoshelter.com
displacement copper silver nitrate chemistry Fundamental Photographs When A Copper Wire Is Placed In Silver Nitrate As soon as the copper metal is added, silver metal begins to. Within minutes, the wire begins to look fuzzy or furry as small silver. A single displacement reaction occurs also known as a redox reaction. When copper wire is placed in silver nitrate solution; When copper is dipped in the solution of silver nitrate, the solution turns blue. Metallic. When A Copper Wire Is Placed In Silver Nitrate.
From www.sciencephoto.com
Copper reacting with silver nitrate Stock Image C030/8167 Science When A Copper Wire Is Placed In Silver Nitrate A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. A single displacement reaction occurs also known as a redox reaction. Metallic copper is placed in a solution of silver nitrate. Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (figure. When A Copper Wire Is Placed In Silver Nitrate.
From www.toppr.com
Vidisha placed a copper wire in silver nitrate solution as shown in the When A Copper Wire Is Placed In Silver Nitrate As soon as the copper metal is added, silver metal begins to. A single displacement reaction occurs also known as a redox reaction. Copper displaces silver from silver nitrate to form silver metal. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Silver metal forms at the surface. When A Copper Wire Is Placed In Silver Nitrate.
From www.sciencephoto.com
Copper reacting with silver nitrate Stock Image A500/0655 Science When A Copper Wire Is Placed In Silver Nitrate Silver metal forms at the surface of the copper metal. Copper displaces silver from silver nitrate to form silver metal. Copper nitrate is seen turning the. When copper wire is placed in silver nitrate solution; Metallic copper is placed in a solution of silver nitrate. (i) when copper wire is placed in silver nitrate solution then the solution becomes blue. When A Copper Wire Is Placed In Silver Nitrate.
From fphoto.photoshelter.com
science chemistry copper nitrate Fundamental Photographs The Art of When A Copper Wire Is Placed In Silver Nitrate We will use electrochemical series to solve the. A single displacement reaction occurs also known as a redox reaction. Silver metal forms at the surface of the copper metal. Copper nitrate is seen turning the. Copper displaces silver from silver nitrate to form silver metal. (i) when copper wire is placed in silver nitrate solution then the solution becomes blue. When A Copper Wire Is Placed In Silver Nitrate.