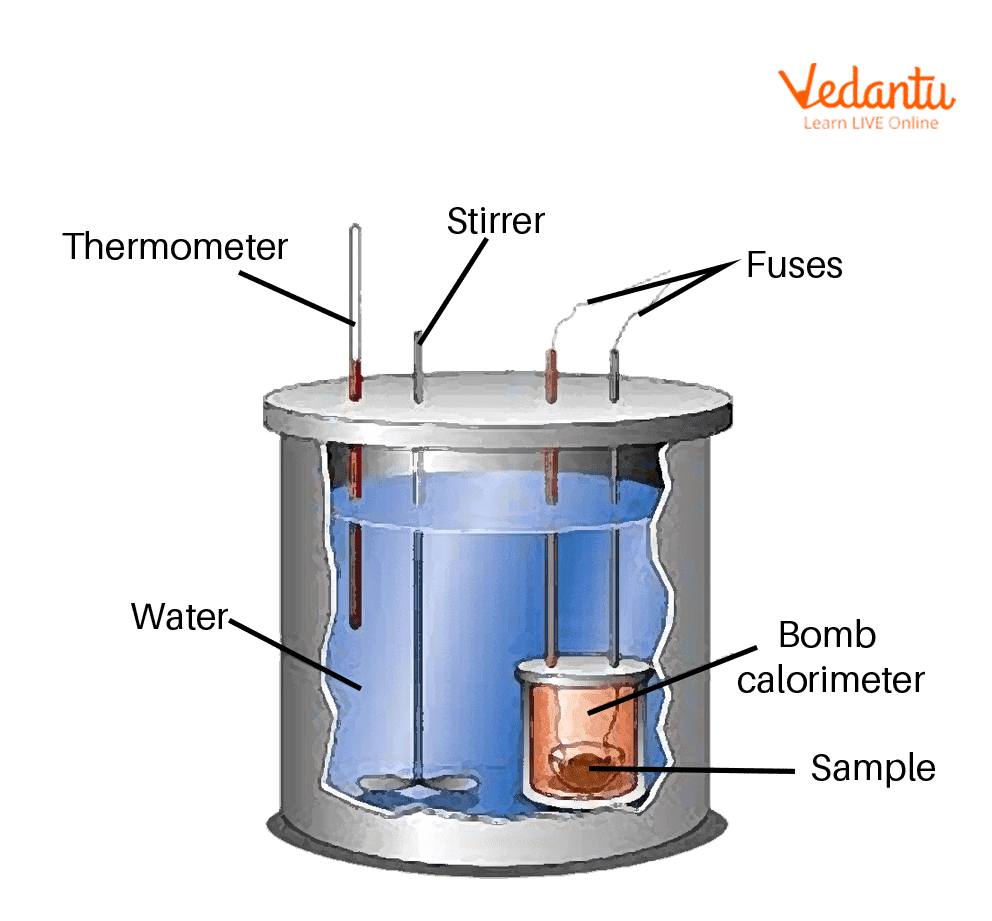
How Does A Calorimeter Work Chemistry . Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. To calculate any changes in enthalpy per mole of a reactant or product the following relationship can be used: The bomb calorimeter has an enclosure in which the reaction happens To do so, the heat is exchanged with a calibrated object. To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. From the first law we can state. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances.
from www.vedantu.com
A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. The bomb calorimeter has an enclosure in which the reaction happens Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance. From the first law we can state. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. To calculate any changes in enthalpy per mole of a reactant or product the following relationship can be used: To do so, the heat is exchanged with a calibrated object.
Bomb Calorimeter Learn Important Terms and Concepts
How Does A Calorimeter Work Chemistry Calorimetry is used to measure amounts of heat transferred to or from a substance. From the first law we can state. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. To calculate any changes in enthalpy per mole of a reactant or product the following relationship can be used: The bomb calorimeter has an enclosure in which the reaction happens Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance.
From people.chem.umass.edu
to Adobe GoLive 6 How Does A Calorimeter Work Chemistry To do so, the heat is exchanged. To do so, the heat is exchanged with a calibrated object. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. From the first law we can state. Calorimetry is used to measure amounts of heat transferred to or from a substance. To calculate any changes in. How Does A Calorimeter Work Chemistry.
From classnotes.org.in
Measurement Of Change In Internal Energy and Enthalpy Chemistry How Does A Calorimeter Work Chemistry A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. To do so, the heat is exchanged. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. From the first law we can state. Calorimetry is used. How Does A Calorimeter Work Chemistry.
From www.thoughtco.com
Calorimeter Definition in Chemistry How Does A Calorimeter Work Chemistry The bomb calorimeter has an enclosure in which the reaction happens Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. Calorimetry is used to measure amounts of heat transferred to or from a substance. To calculate any changes in enthalpy per. How Does A Calorimeter Work Chemistry.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts How Does A Calorimeter Work Chemistry From the first law we can state. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Calorimetry is used to measure amounts of heat transferred to or from a substance. To calculate any changes in enthalpy per mole of a reactant or product the following relationship can be used: The bomb calorimeter has. How Does A Calorimeter Work Chemistry.
From ar.inspiredpencil.com
Simple Bomb Calorimeter How Does A Calorimeter Work Chemistry From the first law we can state. The bomb calorimeter has an enclosure in which the reaction happens Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. To calculate any changes in enthalpy. How Does A Calorimeter Work Chemistry.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 How Does A Calorimeter Work Chemistry To do so, the heat is exchanged with a calibrated object. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is used to measure amounts of heat transferred to or from a substance. A simple calorimeter just consists of a thermometer attached to a metal container. How Does A Calorimeter Work Chemistry.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube How Does A Calorimeter Work Chemistry To calculate any changes in enthalpy per mole of a reactant or product the following relationship can be used: Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. To do so, the heat is exchanged. Calorimetery is an application. How Does A Calorimeter Work Chemistry.
From www.pinterest.com.au
Pin on Science Friday How Does A Calorimeter Work Chemistry From the first law we can state. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. To do so, the heat is exchanged with a calibrated object. The bomb calorimeter has an enclosure in which the reaction happens Calorimetery is an application of the first law of. How Does A Calorimeter Work Chemistry.
From users.highland.edu
Calorimetry How Does A Calorimeter Work Chemistry From the first law we can state. To calculate any changes in enthalpy per mole of a reactant or product the following relationship can be used: Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. The bomb calorimeter has an enclosure in which the reaction happens To do so, the heat is exchanged.. How Does A Calorimeter Work Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How Does A Calorimeter Work Chemistry The bomb calorimeter has an enclosure in which the reaction happens Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. To do so, the heat is exchanged. From the first law we can state. Calorimetry is used to measure amounts of. How Does A Calorimeter Work Chemistry.
From shaunmwilliams.com
Chapter 6 Presentation How Does A Calorimeter Work Chemistry To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance. To calculate any changes in enthalpy per mole of a reactant or product the following relationship can be used: Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is. How Does A Calorimeter Work Chemistry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry How Does A Calorimeter Work Chemistry Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance. To calculate any changes in enthalpy per mole of a reactant. How Does A Calorimeter Work Chemistry.
From www.animalia-life.club
Calorimeter Diagram How Does A Calorimeter Work Chemistry To do so, the heat is exchanged with a calibrated object. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. The bomb calorimeter has an. How Does A Calorimeter Work Chemistry.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson How Does A Calorimeter Work Chemistry Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. From the first law we can state. To do so, the. How Does A Calorimeter Work Chemistry.
From www.slideserve.com
PPT a “ Calorimeter ” PowerPoint Presentation, free download ID7050684 How Does A Calorimeter Work Chemistry To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical. How Does A Calorimeter Work Chemistry.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download How Does A Calorimeter Work Chemistry Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. To. How Does A Calorimeter Work Chemistry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 How Does A Calorimeter Work Chemistry The bomb calorimeter has an enclosure in which the reaction happens Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. A simple calorimeter just consists of a. How Does A Calorimeter Work Chemistry.
From www.nist.gov
SchematicDrawingConeCalorimeter How Does A Calorimeter Work Chemistry Calorimetry is used to measure amounts of heat transferred to or from a substance. From the first law we can state. To do so, the heat is exchanged with a calibrated object. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. Calorimetry describes a set of techniques employed. How Does A Calorimeter Work Chemistry.
From ddscalorimeters.com
How does a bomb calorimeter work? How Does A Calorimeter Work Chemistry Calorimetry is used to measure amounts of heat transferred to or from a substance. The bomb calorimeter has an enclosure in which the reaction happens Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. To do so, the heat is exchanged.. How Does A Calorimeter Work Chemistry.
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter How Does A Calorimeter Work Chemistry To do so, the heat is exchanged with a calibrated object. To calculate any changes in enthalpy per mole of a reactant or product the following relationship can be used: To do so, the heat is exchanged. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. Calorimetery is. How Does A Calorimeter Work Chemistry.
From drivenheisenberg.blogspot.com
Which Diagram Is A Bomb Calorimeter Drivenheisenberg How Does A Calorimeter Work Chemistry The bomb calorimeter has an enclosure in which the reaction happens Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to. How Does A Calorimeter Work Chemistry.
From www.learner.org
The Energy in Chemical Reactions Thermodynamics and Enthalpy How Does A Calorimeter Work Chemistry To do so, the heat is exchanged with a calibrated object. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. Calorimetry is used to measure amounts of heat transferred to or from a substance. The bomb calorimeter has an enclosure in. How Does A Calorimeter Work Chemistry.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry How Does A Calorimeter Work Chemistry To calculate any changes in enthalpy per mole of a reactant or product the following relationship can be used: The bomb calorimeter has an enclosure in which the reaction happens Calorimetry is used to measure amounts of heat transferred to or from a substance. A simple calorimeter just consists of a thermometer attached to a metal container full of water. How Does A Calorimeter Work Chemistry.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby How Does A Calorimeter Work Chemistry Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry describes a set of techniques employed to measure enthalpy. How Does A Calorimeter Work Chemistry.
From wiringdiagram99.blogspot.com
Which Diagram Is A Bomb Calorimeter Wiring Diagram Database How Does A Calorimeter Work Chemistry To do so, the heat is exchanged with a calibrated object. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is used to measure amounts of heat transferred to or from a substance. A simple calorimeter just consists of a thermometer attached to a metal container. How Does A Calorimeter Work Chemistry.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation How Does A Calorimeter Work Chemistry Calorimetry is used to measure amounts of heat transferred to or from a substance. To calculate any changes in enthalpy per mole of a reactant or product the following relationship can be used: To do so, the heat is exchanged. From the first law we can state. Calorimetery is an application of the first law of thermodynamics to heat transfer,. How Does A Calorimeter Work Chemistry.
From saylordotorg.github.io
Calorimetry How Does A Calorimeter Work Chemistry Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. To do so, the heat is exchanged with a calibrated object. A simple calorimeter just consists. How Does A Calorimeter Work Chemistry.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals How Does A Calorimeter Work Chemistry Calorimetry is used to measure amounts of heat transferred to or from a substance. The bomb calorimeter has an enclosure in which the reaction happens To do so, the heat is exchanged. To do so, the heat is exchanged with a calibrated object. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to. How Does A Calorimeter Work Chemistry.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube How Does A Calorimeter Work Chemistry To do so, the heat is exchanged with a calibrated object. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The bomb calorimeter has an enclosure in which the reaction happens A simple calorimeter just consists of a thermometer attached to a metal container full of water. How Does A Calorimeter Work Chemistry.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals How Does A Calorimeter Work Chemistry To calculate any changes in enthalpy per mole of a reactant or product the following relationship can be used: Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Calorimetery is an application of the first law of thermodynamics to. How Does A Calorimeter Work Chemistry.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide How Does A Calorimeter Work Chemistry To do so, the heat is exchanged with a calibrated object. The bomb calorimeter has an enclosure in which the reaction happens Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction. How Does A Calorimeter Work Chemistry.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry How Does A Calorimeter Work Chemistry To calculate any changes in enthalpy per mole of a reactant or product the following relationship can be used: Calorimetry is used to measure amounts of heat transferred to or from a substance. The bomb calorimeter has an enclosure in which the reaction happens To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred. How Does A Calorimeter Work Chemistry.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica How Does A Calorimeter Work Chemistry Calorimetry is used to measure amounts of heat transferred to or from a substance. The bomb calorimeter has an enclosure in which the reaction happens To do so, the heat is exchanged with a calibrated object. To do so, the heat is exchanged. From the first law we can state. Calorimetry is used to measure amounts of heat transferred to. How Does A Calorimeter Work Chemistry.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure How Does A Calorimeter Work Chemistry From the first law we can state. The bomb calorimeter has an enclosure in which the reaction happens To do so, the heat is exchanged with a calibrated object. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices. Calorimetry is used to measure amounts of heat transferred to or from a substance. To. How Does A Calorimeter Work Chemistry.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure How Does A Calorimeter Work Chemistry Calorimetry is used to measure amounts of heat transferred to or from a substance. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat. How Does A Calorimeter Work Chemistry.