E=Hc/Lambda Solve For Lambda . Is there a proof for the equation of photon energy: There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the equation: If we solve the schrodinger. E = h c λ. Λ = (hc)/e = (6.626 × 10⁻³⁴j·s × 2.998 ×. How do i use the equation e = hc/λ to find the wavelength of light. $e = \frac{(h \cdot c)}{\lambda}$? For example, what is the wavelength of a photon that has an energy of 3.36 × 10⁻¹⁹ j? Using e = hc/λ to find wavelength of light emitted 1. A slightly different way would be to use eλ = hc (with the wavelength in meters) and solve for e, then multiply the answer times avogadro's. In this article, we will teach you how to use e= hc/λ also written as e= hc/wavelength or e= hc/lambda to solve plenty of numerical. The teacher at school taught us that there's $e = h*f$, when i told him about $e = \frac{(h \cdot. The expression for the energy of a photon is related to the energy of a simple harmonic oscillator.
from dsp.stackexchange.com
How do i use the equation e = hc/λ to find the wavelength of light. There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the equation: Using e = hc/λ to find wavelength of light emitted 1. $e = \frac{(h \cdot c)}{\lambda}$? In this article, we will teach you how to use e= hc/λ also written as e= hc/wavelength or e= hc/lambda to solve plenty of numerical. The expression for the energy of a photon is related to the energy of a simple harmonic oscillator. E = h c λ. If we solve the schrodinger. For example, what is the wavelength of a photon that has an energy of 3.36 × 10⁻¹⁹ j? Λ = (hc)/e = (6.626 × 10⁻³⁴j·s × 2.998 ×.
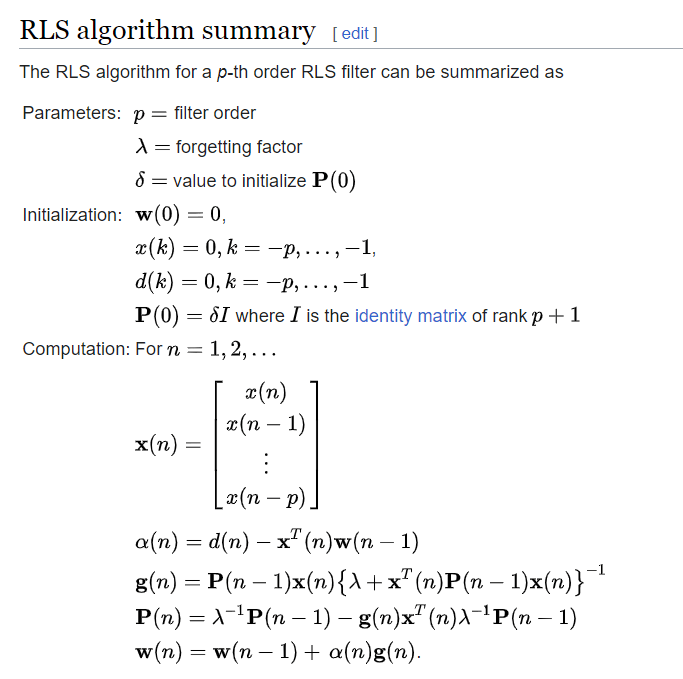
python Convergence of the RLS Algorithm for a Factor \lambda
E=Hc/Lambda Solve For Lambda In this article, we will teach you how to use e= hc/λ also written as e= hc/wavelength or e= hc/lambda to solve plenty of numerical. Λ = (hc)/e = (6.626 × 10⁻³⁴j·s × 2.998 ×. There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the equation: Using e = hc/λ to find wavelength of light emitted 1. $e = \frac{(h \cdot c)}{\lambda}$? A slightly different way would be to use eλ = hc (with the wavelength in meters) and solve for e, then multiply the answer times avogadro's. The expression for the energy of a photon is related to the energy of a simple harmonic oscillator. If we solve the schrodinger. In this article, we will teach you how to use e= hc/λ also written as e= hc/wavelength or e= hc/lambda to solve plenty of numerical. For example, what is the wavelength of a photon that has an energy of 3.36 × 10⁻¹⁹ j? The teacher at school taught us that there's $e = h*f$, when i told him about $e = \frac{(h \cdot. Is there a proof for the equation of photon energy: E = h c λ. How do i use the equation e = hc/λ to find the wavelength of light.
From www.chegg.com
Solved Let X follow the Poisson probability distribution E=Hc/Lambda Solve For Lambda The teacher at school taught us that there's $e = h*f$, when i told him about $e = \frac{(h \cdot. In this article, we will teach you how to use e= hc/λ also written as e= hc/wavelength or e= hc/lambda to solve plenty of numerical. Using e = hc/λ to find wavelength of light emitted 1. A slightly different way. E=Hc/Lambda Solve For Lambda.
From www.doubtnut.com
The energy of a photon is given by E = (hc)/(lambda) If lambda=4xx1 E=Hc/Lambda Solve For Lambda E = h c λ. $e = \frac{(h \cdot c)}{\lambda}$? How do i use the equation e = hc/λ to find the wavelength of light. The expression for the energy of a photon is related to the energy of a simple harmonic oscillator. The teacher at school taught us that there's $e = h*f$, when i told him about $e. E=Hc/Lambda Solve For Lambda.
From www.doubtnut.com
If the energy of a photon is given by E =(hc)/(lambda) , where h is E=Hc/Lambda Solve For Lambda If we solve the schrodinger. Λ = (hc)/e = (6.626 × 10⁻³⁴j·s × 2.998 ×. The expression for the energy of a photon is related to the energy of a simple harmonic oscillator. A slightly different way would be to use eλ = hc (with the wavelength in meters) and solve for e, then multiply the answer times avogadro's. How. E=Hc/Lambda Solve For Lambda.
From lavelle.chem.ucla.edu
Mass of Atoms to Energy CHEMISTRY COMMUNITY E=Hc/Lambda Solve For Lambda The expression for the energy of a photon is related to the energy of a simple harmonic oscillator. There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the equation: How do i use the equation e = hc/λ to find the wavelength of light. $e = \frac{(h \cdot. E=Hc/Lambda Solve For Lambda.
From brainly.pl
Przekształć wzór, tak żeby mieć wzór na lambdę E=hc/lambda Brainly.pl E=Hc/Lambda Solve For Lambda How do i use the equation e = hc/λ to find the wavelength of light. $e = \frac{(h \cdot c)}{\lambda}$? The expression for the energy of a photon is related to the energy of a simple harmonic oscillator. A slightly different way would be to use eλ = hc (with the wavelength in meters) and solve for e, then multiply. E=Hc/Lambda Solve For Lambda.
From www.chegg.com
Solved Find the following derivatives d(yz)/dx, where y = E=Hc/Lambda Solve For Lambda Using e = hc/λ to find wavelength of light emitted 1. The expression for the energy of a photon is related to the energy of a simple harmonic oscillator. The teacher at school taught us that there's $e = h*f$, when i told him about $e = \frac{(h \cdot. In this article, we will teach you how to use e=. E=Hc/Lambda Solve For Lambda.
From byjus.com
How to calculate the lambda maximum value of different series of E=Hc/Lambda Solve For Lambda If we solve the schrodinger. $e = \frac{(h \cdot c)}{\lambda}$? For example, what is the wavelength of a photon that has an energy of 3.36 × 10⁻¹⁹ j? Λ = (hc)/e = (6.626 × 10⁻³⁴j·s × 2.998 ×. There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the. E=Hc/Lambda Solve For Lambda.
From www.chegg.com
Solved Starting from Equation (2.4) derive both Equations E=Hc/Lambda Solve For Lambda In this article, we will teach you how to use e= hc/λ also written as e= hc/wavelength or e= hc/lambda to solve plenty of numerical. A slightly different way would be to use eλ = hc (with the wavelength in meters) and solve for e, then multiply the answer times avogadro's. There is an inverse relationship between the energy of. E=Hc/Lambda Solve For Lambda.
From www.chegg.com
Solved The planck distribution for blackbody radiation can E=Hc/Lambda Solve For Lambda In this article, we will teach you how to use e= hc/λ also written as e= hc/wavelength or e= hc/lambda to solve plenty of numerical. A slightly different way would be to use eλ = hc (with the wavelength in meters) and solve for e, then multiply the answer times avogadro's. If we solve the schrodinger. The teacher at school. E=Hc/Lambda Solve For Lambda.
From dsp.stackexchange.com
python Convergence of the RLS Algorithm for a Factor \lambda E=Hc/Lambda Solve For Lambda A slightly different way would be to use eλ = hc (with the wavelength in meters) and solve for e, then multiply the answer times avogadro's. There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the equation: In this article, we will teach you how to use e=. E=Hc/Lambda Solve For Lambda.
From www.youtube.com
Using E = hc/λ to Calculate Energy of a Wave (Example 4) YouTube E=Hc/Lambda Solve For Lambda A slightly different way would be to use eλ = hc (with the wavelength in meters) and solve for e, then multiply the answer times avogadro's. If we solve the schrodinger. For example, what is the wavelength of a photon that has an energy of 3.36 × 10⁻¹⁹ j? In this article, we will teach you how to use e=. E=Hc/Lambda Solve For Lambda.
From www.youtube.com
Wave Math E=hc/lambda example YouTube E=Hc/Lambda Solve For Lambda Using e = hc/λ to find wavelength of light emitted 1. If we solve the schrodinger. The teacher at school taught us that there's $e = h*f$, when i told him about $e = \frac{(h \cdot. Λ = (hc)/e = (6.626 × 10⁻³⁴j·s × 2.998 ×. In this article, we will teach you how to use e= hc/λ also written. E=Hc/Lambda Solve For Lambda.
From www.numerade.com
SOLVED If λ0 and λ the threshold wavelength and the wavelength of E=Hc/Lambda Solve For Lambda Λ = (hc)/e = (6.626 × 10⁻³⁴j·s × 2.998 ×. How do i use the equation e = hc/λ to find the wavelength of light. E = h c λ. Is there a proof for the equation of photon energy: For example, what is the wavelength of a photon that has an energy of 3.36 × 10⁻¹⁹ j? Using e. E=Hc/Lambda Solve For Lambda.
From www.toppr.com
Solve [a̅ × b̅b̅ × c̅c̅ × a̅] = lambda [a̅b̅c̅] then lambda E=Hc/Lambda Solve For Lambda How do i use the equation e = hc/λ to find the wavelength of light. Is there a proof for the equation of photon energy: A slightly different way would be to use eλ = hc (with the wavelength in meters) and solve for e, then multiply the answer times avogadro's. If we solve the schrodinger. Using e = hc/λ. E=Hc/Lambda Solve For Lambda.
From www.toppr.com
Find the value of lambda for which the homogeneous system of equations E=Hc/Lambda Solve For Lambda Is there a proof for the equation of photon energy: If we solve the schrodinger. Using e = hc/λ to find wavelength of light emitted 1. For example, what is the wavelength of a photon that has an energy of 3.36 × 10⁻¹⁹ j? There is an inverse relationship between the energy of a photon (e) and the wavelength of. E=Hc/Lambda Solve For Lambda.
From www.youtube.com
Using E = hc/λ (Example 3) YouTube E=Hc/Lambda Solve For Lambda The teacher at school taught us that there's $e = h*f$, when i told him about $e = \frac{(h \cdot. The expression for the energy of a photon is related to the energy of a simple harmonic oscillator. Is there a proof for the equation of photon energy: A slightly different way would be to use eλ = hc (with. E=Hc/Lambda Solve For Lambda.
From www.chegg.com
Solved For each B, lambda elementof R, the function u(x, t) E=Hc/Lambda Solve For Lambda Λ = (hc)/e = (6.626 × 10⁻³⁴j·s × 2.998 ×. How do i use the equation e = hc/λ to find the wavelength of light. The teacher at school taught us that there's $e = h*f$, when i told him about $e = \frac{(h \cdot. The expression for the energy of a photon is related to the energy of a. E=Hc/Lambda Solve For Lambda.
From www.toppr.com
Solve for lambda if a^2 + lambda ab ac ab b^2 + lambda bc ac bc c^2 E=Hc/Lambda Solve For Lambda Λ = (hc)/e = (6.626 × 10⁻³⁴j·s × 2.998 ×. How do i use the equation e = hc/λ to find the wavelength of light. E = h c λ. Using e = hc/λ to find wavelength of light emitted 1. For example, what is the wavelength of a photon that has an energy of 3.36 × 10⁻¹⁹ j? The. E=Hc/Lambda Solve For Lambda.
From www.chegg.com
Solved * = RH (1 1) Where (Greek letter lambda) is the E=Hc/Lambda Solve For Lambda E = h c λ. In this article, we will teach you how to use e= hc/λ also written as e= hc/wavelength or e= hc/lambda to solve plenty of numerical. Is there a proof for the equation of photon energy: Using e = hc/λ to find wavelength of light emitted 1. There is an inverse relationship between the energy of. E=Hc/Lambda Solve For Lambda.
From www.youtube.com
Teach Demonstrating E=hc/lambda with cc YouTube E=Hc/Lambda Solve For Lambda Is there a proof for the equation of photon energy: Using e = hc/λ to find wavelength of light emitted 1. In this article, we will teach you how to use e= hc/λ also written as e= hc/wavelength or e= hc/lambda to solve plenty of numerical. The teacher at school taught us that there's $e = h*f$, when i told. E=Hc/Lambda Solve For Lambda.
From www.gauthmath.com
Solved Determine the energy of a photon with a wavelength of 3.64* 10 E=Hc/Lambda Solve For Lambda If we solve the schrodinger. Is there a proof for the equation of photon energy: There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the equation: A slightly different way would be to use eλ = hc (with the wavelength in meters) and solve for e, then multiply. E=Hc/Lambda Solve For Lambda.
From www.toppr.com
If lambda1 and lambda2 are the two values of lambda such that the roots E=Hc/Lambda Solve For Lambda Using e = hc/λ to find wavelength of light emitted 1. There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the equation: How do i use the equation e = hc/λ to find the wavelength of light. $e = \frac{(h \cdot c)}{\lambda}$? Is there a proof for the. E=Hc/Lambda Solve For Lambda.
From www.chegg.com
Solved The Planck function radiance is B_lambda (T) = E=Hc/Lambda Solve For Lambda In this article, we will teach you how to use e= hc/λ also written as e= hc/wavelength or e= hc/lambda to solve plenty of numerical. There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the equation: A slightly different way would be to use eλ = hc (with. E=Hc/Lambda Solve For Lambda.
From www.investopedia.com
Lambda Definition E=Hc/Lambda Solve For Lambda In this article, we will teach you how to use e= hc/λ also written as e= hc/wavelength or e= hc/lambda to solve plenty of numerical. E = h c λ. For example, what is the wavelength of a photon that has an energy of 3.36 × 10⁻¹⁹ j? Is there a proof for the equation of photon energy: If we. E=Hc/Lambda Solve For Lambda.
From blog.sciencenet.cn
科学网—[请教] 《物理学》里光子(光量子)能量 E=hν 精确成立的条件是什么? 杨正瓴的博文 E=Hc/Lambda Solve For Lambda If we solve the schrodinger. Using e = hc/λ to find wavelength of light emitted 1. For example, what is the wavelength of a photon that has an energy of 3.36 × 10⁻¹⁹ j? How do i use the equation e = hc/λ to find the wavelength of light. The expression for the energy of a photon is related to. E=Hc/Lambda Solve For Lambda.
From wiki.cs.money
Lambda Pin — CSGO/CS2 Wiki by CS.MONEY E=Hc/Lambda Solve For Lambda The teacher at school taught us that there's $e = h*f$, when i told him about $e = \frac{(h \cdot. A slightly different way would be to use eλ = hc (with the wavelength in meters) and solve for e, then multiply the answer times avogadro's. There is an inverse relationship between the energy of a photon (e) and the. E=Hc/Lambda Solve For Lambda.
From www.doubtnut.com
The units for equation lambda = (h)/(mv) are E=Hc/Lambda Solve For Lambda Is there a proof for the equation of photon energy: A slightly different way would be to use eλ = hc (with the wavelength in meters) and solve for e, then multiply the answer times avogadro's. The teacher at school taught us that there's $e = h*f$, when i told him about $e = \frac{(h \cdot. $e = \frac{(h \cdot. E=Hc/Lambda Solve For Lambda.
From www.chegg.com
Solved I = 2 pi hc^2/lambda^5(e^hc/lambda k_B T 1) dI/d E=Hc/Lambda Solve For Lambda The teacher at school taught us that there's $e = h*f$, when i told him about $e = \frac{(h \cdot. Is there a proof for the equation of photon energy: E = h c λ. How do i use the equation e = hc/λ to find the wavelength of light. For example, what is the wavelength of a photon that. E=Hc/Lambda Solve For Lambda.
From towardsdatascience.com
How to Effectively Use Lambda Functions in Python as a Data Scientist E=Hc/Lambda Solve For Lambda There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the equation: In this article, we will teach you how to use e= hc/λ also written as e= hc/wavelength or e= hc/lambda to solve plenty of numerical. Is there a proof for the equation of photon energy: $e =. E=Hc/Lambda Solve For Lambda.
From www.numerade.com
SOLVED(a) Find the wavelength of a 4.1 eV photon. In what part of the E=Hc/Lambda Solve For Lambda Λ = (hc)/e = (6.626 × 10⁻³⁴j·s × 2.998 ×. E = h c λ. There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the equation: The expression for the energy of a photon is related to the energy of a simple harmonic oscillator. The teacher at school. E=Hc/Lambda Solve For Lambda.
From byjus.com
Why E= 1/2 mv2 not applied for photon and why E= hc/lambda not applied E=Hc/Lambda Solve For Lambda Λ = (hc)/e = (6.626 × 10⁻³⁴j·s × 2.998 ×. The teacher at school taught us that there's $e = h*f$, when i told him about $e = \frac{(h \cdot. There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the equation: E = h c λ. For example,. E=Hc/Lambda Solve For Lambda.
From www.doubtnut.com
When radiations of wavelength lambda(1), lambda(2) (= 0.6 lambda(1)), E=Hc/Lambda Solve For Lambda The expression for the energy of a photon is related to the energy of a simple harmonic oscillator. Using e = hc/λ to find wavelength of light emitted 1. E = h c λ. How do i use the equation e = hc/λ to find the wavelength of light. $e = \frac{(h \cdot c)}{\lambda}$? For example, what is the wavelength. E=Hc/Lambda Solve For Lambda.
From www.chegg.com
Solved E = hf = hc/lambda p = h/lambda The "BLUE" opsin E=Hc/Lambda Solve For Lambda In this article, we will teach you how to use e= hc/λ also written as e= hc/wavelength or e= hc/lambda to solve plenty of numerical. There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the equation: If we solve the schrodinger. Using e = hc/λ to find wavelength. E=Hc/Lambda Solve For Lambda.
From www.chegg.com
Solved Eigenvalue PDE? How can I solve for lambda of the new E=Hc/Lambda Solve For Lambda Λ = (hc)/e = (6.626 × 10⁻³⁴j·s × 2.998 ×. There is an inverse relationship between the energy of a photon (e) and the wavelength of the light (λ) given by the equation: For example, what is the wavelength of a photon that has an energy of 3.36 × 10⁻¹⁹ j? Using e = hc/λ to find wavelength of light. E=Hc/Lambda Solve For Lambda.
From scoop.eduncle.com
Why is it lambda square. isnt e=hc/lambda? E=Hc/Lambda Solve For Lambda Using e = hc/λ to find wavelength of light emitted 1. Λ = (hc)/e = (6.626 × 10⁻³⁴j·s × 2.998 ×. The expression for the energy of a photon is related to the energy of a simple harmonic oscillator. Is there a proof for the equation of photon energy: The teacher at school taught us that there's $e = h*f$,. E=Hc/Lambda Solve For Lambda.