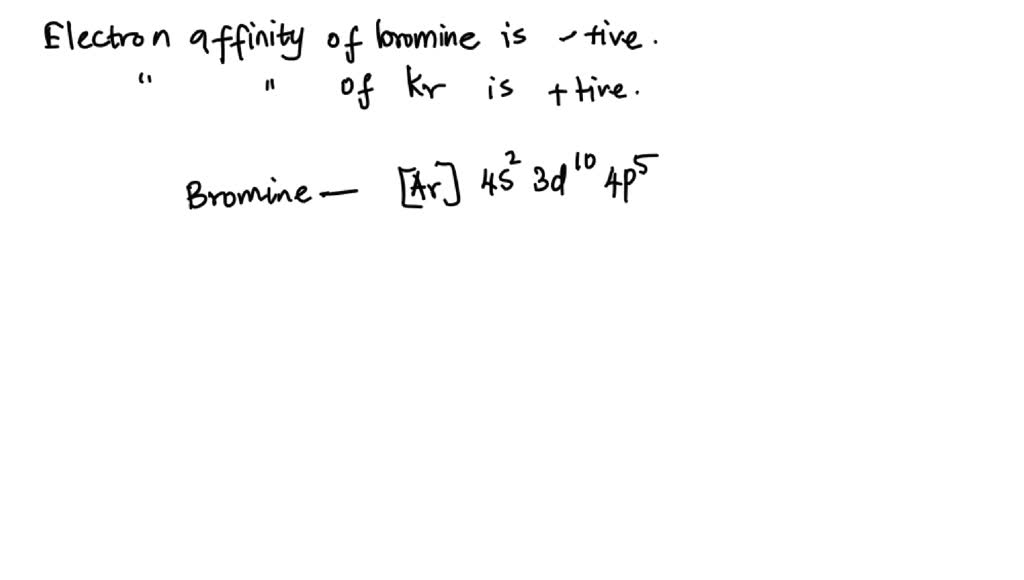Electron Affinity Krypton Bromine . electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. compare krypton and bromine on the basis of their properties, attributes and periodic table facts. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.
from www.numerade.com
electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. compare krypton and bromine on the basis of their properties, attributes and periodic table facts. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.
SOLVED While the electron affinity of bromine is a negative quantity
Electron Affinity Krypton Bromine In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. compare krypton and bromine on the basis of their properties, attributes and periodic table facts. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right.
From material-properties.org
Krypton Periodic Table and Atomic Properties Electron Affinity Krypton Bromine electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is defined as the change in energy (in. Electron Affinity Krypton Bromine.
From sciencenotes.org
Krypton Atom Science Notes and Projects Electron Affinity Krypton Bromine In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In general, elements with the most negative electron affinities (the highest affinity for an added electron). Electron Affinity Krypton Bromine.
From exobbalij.blob.core.windows.net
Bromine Atom Electrons at Paula McCullough blog Electron Affinity Krypton Bromine electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. . Electron Affinity Krypton Bromine.
From www.shutterstock.com
Bohr Model Krypton Atom Electron Structure Stock Vector (Royalty Free Electron Affinity Krypton Bromine electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. . Electron Affinity Krypton Bromine.
From pubchem.ncbi.nlm.nih.gov
Electron Affinity Periodic Table of Elements PubChem Electron Affinity Krypton Bromine In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In. Electron Affinity Krypton Bromine.
From infolkcomunitario.blogspot.com
Valence Krypton Electron Configuration elements Periodic Table Electron Affinity Krypton Bromine electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is the amount of energy change (δe) that. Electron Affinity Krypton Bromine.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Electron Affinity Krypton Bromine In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. . Electron Affinity Krypton Bromine.
From www.sciencephoto.com
Krypton, atomic structure Stock Image C018/3717 Science Photo Library Electron Affinity Krypton Bromine 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In other words, when the electron is added to. Electron Affinity Krypton Bromine.
From www.numerade.com
SOLVEDAlthough the electron affinity of bromine is a negative quantity Electron Affinity Krypton Bromine electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. the electron affinity (ea) of an element is. Electron Affinity Krypton Bromine.
From pediabay.com
Electron Affinity Chart of Elements (With Periodic Table) Pediabay Electron Affinity Krypton Bromine electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. In general, elements with the most negative electron affinities (the highest. Electron Affinity Krypton Bromine.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Electron Affinity Krypton Bromine electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. the electron affinity (ea) of an element is. Electron Affinity Krypton Bromine.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Electron Affinity Krypton Bromine In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. electron affinity is the amount of energy change (δe) that occurs when an. Electron Affinity Krypton Bromine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Electron Affinity Krypton Bromine In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. compare krypton and bromine on the basis of their properties, attributes and periodic table facts. electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion.. Electron Affinity Krypton Bromine.
From www.numerade.com
SOLVED While the electron affinity of bromine is a negative quantity Electron Affinity Krypton Bromine electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In general, elements with the most negative electron affinities. Electron Affinity Krypton Bromine.
From chemistry291.blogspot.com
【5 Steps】Electron Configuration for Krypton in Just 5 Steps Electron Affinity Krypton Bromine In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. compare krypton and bromine on the basis of their properties, attributes and periodic table facts. electron affinity refers to the energy released when an additional. Electron Affinity Krypton Bromine.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Electron Affinity Krypton Bromine compare krypton and bromine on the basis of their properties, attributes and periodic table facts. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. electron affinity is the amount of energy change (δe) that occurs when an electron is added. Electron Affinity Krypton Bromine.
From periodictableguide.com
Electron Affinity Chart (Labeled Periodic table + List) Electron Affinity Krypton Bromine electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. electron affinity refers to the energy released when an additional electron is attached to. Electron Affinity Krypton Bromine.
From www.webelements.com
Elements Periodic Table » Bromine » properties of free atoms Electron Affinity Krypton Bromine 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. compare krypton and bromine on the basis of their properties, attributes and periodic table facts. . Electron Affinity Krypton Bromine.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Electron Affinity Krypton Bromine the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. compare krypton and bromine on the basis of their properties, attributes and periodic table facts. electron affinity refers to the energy released when an additional electron is attached to a neutral. Electron Affinity Krypton Bromine.
From www.numerade.com
SOLVED From which atom of each of the following pairs is it easier to Electron Affinity Krypton Bromine the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is defined as the change in. Electron Affinity Krypton Bromine.
From www.animalia-life.club
Electron Configuration For Bromine Electron Affinity Krypton Bromine electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. 125 rows the electron affinity is defined as the. Electron Affinity Krypton Bromine.
From exorwrmka.blob.core.windows.net
Bromine Of Electron Affinity at Curtis Phillips blog Electron Affinity Krypton Bromine electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. compare krypton and bromine on the basis of their properties, attributes and periodic table facts. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the. Electron Affinity Krypton Bromine.
From dxogpncsm.blob.core.windows.net
Bromine Element Protons Neutrons And Electrons at Kelly Moya blog Electron Affinity Krypton Bromine the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. 125 rows the electron affinity is. Electron Affinity Krypton Bromine.
From www.webelements.com
Elements Periodic Table » Bromine » properties of free atoms Electron Affinity Krypton Bromine In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In general, elements with the most negative electron affinities (the highest affinity for an added. Electron Affinity Krypton Bromine.
From www.youtube.com
Krypton Electron Configuration How to Write the Electron Electron Affinity Krypton Bromine electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity refers to the energy released when an. Electron Affinity Krypton Bromine.
From www.pikpng.com
Krypton Electronic Structure Of Bromine Clipart (3601973) PikPng Electron Affinity Krypton Bromine electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. the electron affinity (ea) of an element is the energy. Electron Affinity Krypton Bromine.
From exoedqopw.blob.core.windows.net
Ionization Energy Of Bromine Equation at David Taber blog Electron Affinity Krypton Bromine compare krypton and bromine on the basis of their properties, attributes and periodic table facts. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the. Electron Affinity Krypton Bromine.
From www.schoolmykids.com
Krypton (Kr) Element Information, Facts, Properties, Uses Periodic Electron Affinity Krypton Bromine In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion.. Electron Affinity Krypton Bromine.
From www.animalia-life.club
Atomic Structure Of Krypton Electron Affinity Krypton Bromine In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion. 125. Electron Affinity Krypton Bromine.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Electron Affinity Krypton Bromine the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is the amount of energy change. Electron Affinity Krypton Bromine.
From schematicfixspellers.z22.web.core.windows.net
Bohr Diagram For Krypton Electron Affinity Krypton Bromine electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. . Electron Affinity Krypton Bromine.
From www.youtube.com
Electron Configuration of Krypton Kr Lesson YouTube Electron Affinity Krypton Bromine In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom. Electron Affinity Krypton Bromine.
From periodictable.me
2000pxElectron_configuration_bromine.svg Dynamic Periodic Table of Electron Affinity Krypton Bromine electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity refers to the energy released when an additional. Electron Affinity Krypton Bromine.
From www.youtube.com
Kr electronic configurationHow to write electronic configuration of Electron Affinity Krypton Bromine In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right. the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion.. Electron Affinity Krypton Bromine.
From diagramweb.net
Electron Dot Diagram For Krypton Electron Affinity Krypton Bromine the electron affinity (ea) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion. 125 rows the electron affinity is defined. Electron Affinity Krypton Bromine.