How Does An Enzyme Affect Delta G . a catalyst does not change the free energy i.e. They repeatedly bind substrate, convert, and. It only changes the activation energy. δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction. Instead, they increase reaction rates by lowering the activation energy, δg‡. The change in free energy, δg, is equal to the. ∆g of the net reaction. enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. The catalysed reaction can be. gibbs free energy, denoted g, combines enthalpy and entropy into a single value. enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. δg can be found by subtracting the free energy of the reactants from the free energy of the products. enzymes do not afect δg° rxn;
from www.chegg.com
They repeatedly bind substrate, convert, and. The catalysed reaction can be. enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. a catalyst does not change the free energy i.e. Instead, they increase reaction rates by lowering the activation energy, δg‡. enzymes do not afect δg° rxn; ∆g of the net reaction. gibbs free energy, denoted g, combines enthalpy and entropy into a single value. It only changes the activation energy. δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction.
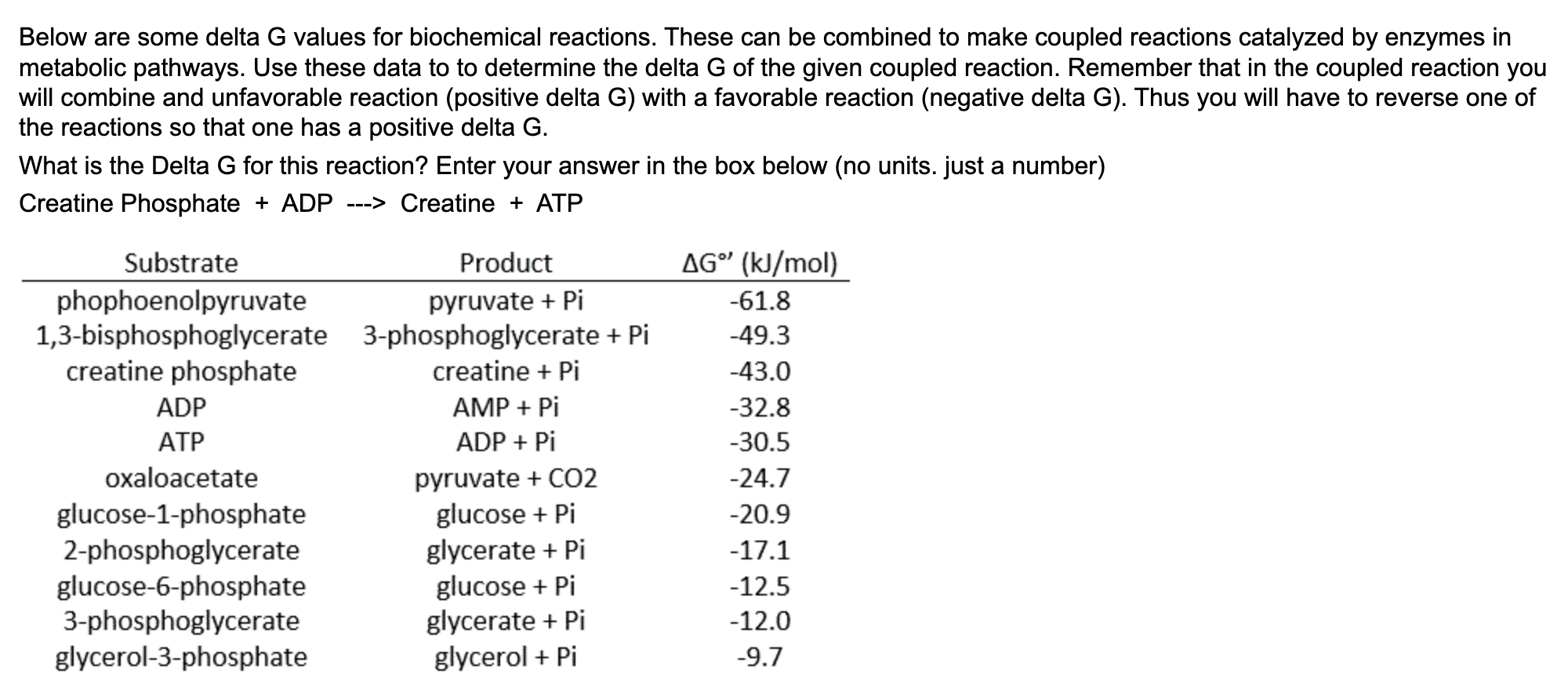
Solved Below are some delta G values for biochemical
How Does An Enzyme Affect Delta G enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. It only changes the activation energy. a catalyst does not change the free energy i.e. enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. The change in free energy, δg, is equal to the. δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction. δg can be found by subtracting the free energy of the reactants from the free energy of the products. Instead, they increase reaction rates by lowering the activation energy, δg‡. enzymes do not afect δg° rxn; They repeatedly bind substrate, convert, and. gibbs free energy, denoted g, combines enthalpy and entropy into a single value. ∆g of the net reaction. enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. The catalysed reaction can be.
From zymvol.com
All you need to know about enzymes Zymvol How Does An Enzyme Affect Delta G ∆g of the net reaction. enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. enzymes do not afect δg° rxn; δg can be found by subtracting the free energy of the reactants from the free energy of the products. They repeatedly bind substrate, convert, and. The catalysed reaction can be. Instead,. How Does An Enzyme Affect Delta G.
From www.biologyonline.com
Enzyme Definition and Examples Biology Online Dictionary How Does An Enzyme Affect Delta G The change in free energy, δg, is equal to the. They repeatedly bind substrate, convert, and. ∆g of the net reaction. enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. The catalysed reaction can be. enzymes do not afect δg° rxn; a catalyst does not change the. How Does An Enzyme Affect Delta G.
From ditki.com
Biochemistry Glossary Enzyme Overview ditki medical & biological How Does An Enzyme Affect Delta G enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. It only changes the activation energy. They repeatedly bind substrate, convert, and. enzymes do not afect δg° rxn; a catalyst does not change the free energy i.e. δg can be found by subtracting the free energy of. How Does An Enzyme Affect Delta G.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry ΔG‡ How Does An Enzyme Affect Delta G The change in free energy, δg, is equal to the. enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. enzymes do not afect δg° rxn; ∆g of the net reaction. They repeatedly bind substrate, convert, and. Instead, they increase reaction rates by lowering the activation energy, δg‡. . How Does An Enzyme Affect Delta G.
From dxofnicyz.blob.core.windows.net
Enzymes Biomolecule Examples at Herbert Morelock blog How Does An Enzyme Affect Delta G Instead, they increase reaction rates by lowering the activation energy, δg‡. δg can be found by subtracting the free energy of the reactants from the free energy of the products. ∆g of the net reaction. gibbs free energy, denoted g, combines enthalpy and entropy into a single value. a catalyst does not change the free energy i.e.. How Does An Enzyme Affect Delta G.
From exoxeaxcj.blob.core.windows.net
Enzymes And Catalysts Reaction at Vincent Miller blog How Does An Enzyme Affect Delta G δg can be found by subtracting the free energy of the reactants from the free energy of the products. enzymes do not afect δg° rxn; The change in free energy, δg, is equal to the. a catalyst does not change the free energy i.e. It only changes the activation energy. Instead, they increase reaction rates by lowering. How Does An Enzyme Affect Delta G.
From www.chegg.com
Solved Below are some delta G values for biochemical How Does An Enzyme Affect Delta G a catalyst does not change the free energy i.e. They repeatedly bind substrate, convert, and. ∆g of the net reaction. δg can be found by subtracting the free energy of the reactants from the free energy of the products. enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of. How Does An Enzyme Affect Delta G.
From biology4ibdp.weebly.com
2.5 Enzymes BIOLOGY4IBDP How Does An Enzyme Affect Delta G enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. Instead, they increase reaction rates by lowering the activation energy, δg‡. The catalysed reaction can be. δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction. ∆g of the net reaction. enzymes decrease the gibbs. How Does An Enzyme Affect Delta G.
From www.thoughtco.com
Structure and Function of an Enzyme How Does An Enzyme Affect Delta G Instead, they increase reaction rates by lowering the activation energy, δg‡. enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. ∆g of the net reaction. The change in free energy, δg, is equal to the. enzymes are biological catalysts, and therefore not consumed or altered by the reactions. How Does An Enzyme Affect Delta G.
From www.slideserve.com
PPT HOW ENZYMES WORK PowerPoint Presentation, free download ID6954410 How Does An Enzyme Affect Delta G The change in free energy, δg, is equal to the. gibbs free energy, denoted g, combines enthalpy and entropy into a single value. a catalyst does not change the free energy i.e. enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. δg can be found by subtracting the free energy. How Does An Enzyme Affect Delta G.
From science.halleyhosting.com
Chapter 8 Enzymes How Does An Enzyme Affect Delta G gibbs free energy, denoted g, combines enthalpy and entropy into a single value. They repeatedly bind substrate, convert, and. It only changes the activation energy. The catalysed reaction can be. enzymes do not afect δg° rxn; δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction. a catalyst does. How Does An Enzyme Affect Delta G.
From exolhjqte.blob.core.windows.net
Enzymes Chemical Pathways at Norma Reynolds blog How Does An Enzyme Affect Delta G δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction. enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. They repeatedly bind substrate, convert, and. enzymes do not afect δg° rxn; enzymes decrease the gibbs free energy of activation, but they have no. How Does An Enzyme Affect Delta G.
From wires.onlinelibrary.wiley.com
Finding the ΔΔG spot Are predictors of binding affinity changes upon How Does An Enzyme Affect Delta G ∆g of the net reaction. a catalyst does not change the free energy i.e. enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. It only changes the activation energy. Instead, they increase reaction rates by lowering the activation energy, δg‡. They repeatedly bind substrate, convert, and. The change. How Does An Enzyme Affect Delta G.
From www.chegg.com
Solved Below are some delta G values for biochemical How Does An Enzyme Affect Delta G enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. a catalyst does not change the free energy i.e. The change in free energy, δg, is equal to the. enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. δg can be. How Does An Enzyme Affect Delta G.
From www.genome.gov
Enzyme How Does An Enzyme Affect Delta G enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. It only changes the activation energy. ∆g of the net reaction. a catalyst does not change the free energy i.e. The change in free energy, δg, is equal to the. enzymes do not afect δg° rxn; enzymes decrease the gibbs free. How Does An Enzyme Affect Delta G.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1940469 How Does An Enzyme Affect Delta G δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction. gibbs free energy, denoted g, combines enthalpy and entropy into a single value. a catalyst does not change the free energy i.e. Instead, they increase reaction rates by lowering the activation energy, δg‡. The change in free energy, δg, is. How Does An Enzyme Affect Delta G.
From www.youtube.com
Chapter 6 pt2 Delta G YouTube How Does An Enzyme Affect Delta G enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction. It only changes the activation energy. The change in free energy, δg, is equal to the. The catalysed reaction can be. They repeatedly bind substrate, convert,. How Does An Enzyme Affect Delta G.
From www.onlinebiologynotes.com
Rate of enzyme reactions and factor affecting the rate of enzyme How Does An Enzyme Affect Delta G enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. It only changes the activation energy. enzymes do not afect δg° rxn; Instead, they increase reaction rates by lowering the activation energy, δg‡.. How Does An Enzyme Affect Delta G.
From www.chegg.com
Solved Question 27 (6 points) The diagram below depicts a How Does An Enzyme Affect Delta G a catalyst does not change the free energy i.e. They repeatedly bind substrate, convert, and. The catalysed reaction can be. The change in free energy, δg, is equal to the. Instead, they increase reaction rates by lowering the activation energy, δg‡. δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction.. How Does An Enzyme Affect Delta G.
From www.biomol.com
Guide to Enzyme Unit Definitions and Assay Design Biomol Blog How Does An Enzyme Affect Delta G The catalysed reaction can be. a catalyst does not change the free energy i.e. gibbs free energy, denoted g, combines enthalpy and entropy into a single value. It only changes the activation energy. enzymes do not afect δg° rxn; enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. δg. How Does An Enzyme Affect Delta G.
From chim.lu
In this case, the free energy difference of Gibbs \Delta \; G How Does An Enzyme Affect Delta G They repeatedly bind substrate, convert, and. enzymes do not afect δg° rxn; δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction. The change in free energy, δg, is equal to the. gibbs free energy, denoted g, combines enthalpy and entropy into a single value. ∆g of the net reaction.. How Does An Enzyme Affect Delta G.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID2187703 How Does An Enzyme Affect Delta G gibbs free energy, denoted g, combines enthalpy and entropy into a single value. enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. Instead, they increase reaction rates by lowering the activation energy, δg‡. a catalyst does not change the free energy i.e. δg can be found. How Does An Enzyme Affect Delta G.
From openoregon.pressbooks.pub
Enzymes Principles of Biology How Does An Enzyme Affect Delta G enzymes do not afect δg° rxn; Instead, they increase reaction rates by lowering the activation energy, δg‡. The change in free energy, δg, is equal to the. It only changes the activation energy. enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. ∆g of the net reaction. They. How Does An Enzyme Affect Delta G.
From www.slideserve.com
PPT Chemical reactions and enzymes PowerPoint Presentation, free How Does An Enzyme Affect Delta G They repeatedly bind substrate, convert, and. Instead, they increase reaction rates by lowering the activation energy, δg‡. ∆g of the net reaction. a catalyst does not change the free energy i.e. δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction. The catalysed reaction can be. The change in free energy,. How Does An Enzyme Affect Delta G.
From www.slideserve.com
PPT Chapter 6 Enzymes PowerPoint Presentation, free download ID5143485 How Does An Enzyme Affect Delta G δg can be found by subtracting the free energy of the reactants from the free energy of the products. The change in free energy, δg, is equal to the. Instead, they increase reaction rates by lowering the activation energy, δg‡. It only changes the activation energy. δg is the overall energy released during the reaction and accounts for. How Does An Enzyme Affect Delta G.
From jumpstarterdiscount.blogspot.com
The Diagram Below Represents A Spontaneous Reaction δg Wiring Diagram How Does An Enzyme Affect Delta G enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. enzymes do not afect δg° rxn; The change in free energy, δg, is equal to the. Instead, they increase reaction rates by lowering the activation energy, δg‡. gibbs free energy, denoted g, combines enthalpy and entropy into a. How Does An Enzyme Affect Delta G.
From www.askiitians.com
Biology Enzymes for NEET (AIPMT) askIITians How Does An Enzyme Affect Delta G enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction. enzymes do not afect δg° rxn; The change in free energy, δg, is equal to the. δg can be found. How Does An Enzyme Affect Delta G.
From www.slideserve.com
PPT Revision lesson on enzymes PowerPoint Presentation, free download How Does An Enzyme Affect Delta G It only changes the activation energy. δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction. Instead, they increase reaction rates by lowering the activation energy, δg‡. enzymes do not afect δg° rxn; enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy. How Does An Enzyme Affect Delta G.
From studygripewater.z21.web.core.windows.net
How To Determine Delta S Chemistry How Does An Enzyme Affect Delta G Instead, they increase reaction rates by lowering the activation energy, δg‡. ∆g of the net reaction. δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction. enzymes do not afect δg° rxn; They repeatedly bind substrate, convert, and. gibbs free energy, denoted g, combines enthalpy and entropy into a single. How Does An Enzyme Affect Delta G.
From www.onlinebiologynotes.com
Enzymes Properties and Mechanism of enzyme action Online Biology Notes How Does An Enzyme Affect Delta G The catalysed reaction can be. enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. They repeatedly bind substrate, convert, and. The change in free energy, δg, is equal to the. enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. δg is. How Does An Enzyme Affect Delta G.
From present5.com
Enzyme Structure classification and mechanism of action How Does An Enzyme Affect Delta G gibbs free energy, denoted g, combines enthalpy and entropy into a single value. It only changes the activation energy. They repeatedly bind substrate, convert, and. enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. The change in free energy, δg, is equal to the. Instead, they increase reaction rates by lowering the. How Does An Enzyme Affect Delta G.
From dxoridtwr.blob.core.windows.net
The Function Of Enzymes In Plants Is at Richard Borchert blog How Does An Enzyme Affect Delta G The change in free energy, δg, is equal to the. enzymes do not afect δg° rxn; ∆g of the net reaction. enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. δg. How Does An Enzyme Affect Delta G.
From dxoamoqwd.blob.core.windows.net
What Organic Compound Is An Enzyme at Francis Johnston blog How Does An Enzyme Affect Delta G enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. a catalyst does not change the free energy i.e. δg can be found by subtracting the free energy of the reactants from the free energy of the products. Instead, they increase reaction rates by lowering the activation energy,. How Does An Enzyme Affect Delta G.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology How Does An Enzyme Affect Delta G gibbs free energy, denoted g, combines enthalpy and entropy into a single value. ∆g of the net reaction. δg is the overall energy released during the reaction and accounts for the equilibrium of the reaction. enzymes are biological catalysts, and therefore not consumed or altered by the reactions they catalyze. The catalysed reaction can be. They repeatedly. How Does An Enzyme Affect Delta G.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1940469 How Does An Enzyme Affect Delta G ∆g of the net reaction. It only changes the activation energy. enzymes decrease the gibbs free energy of activation, but they have no effect on the free energy of reaction. enzymes do not afect δg° rxn; The catalysed reaction can be. The change in free energy, δg, is equal to the. δg is the overall energy released. How Does An Enzyme Affect Delta G.