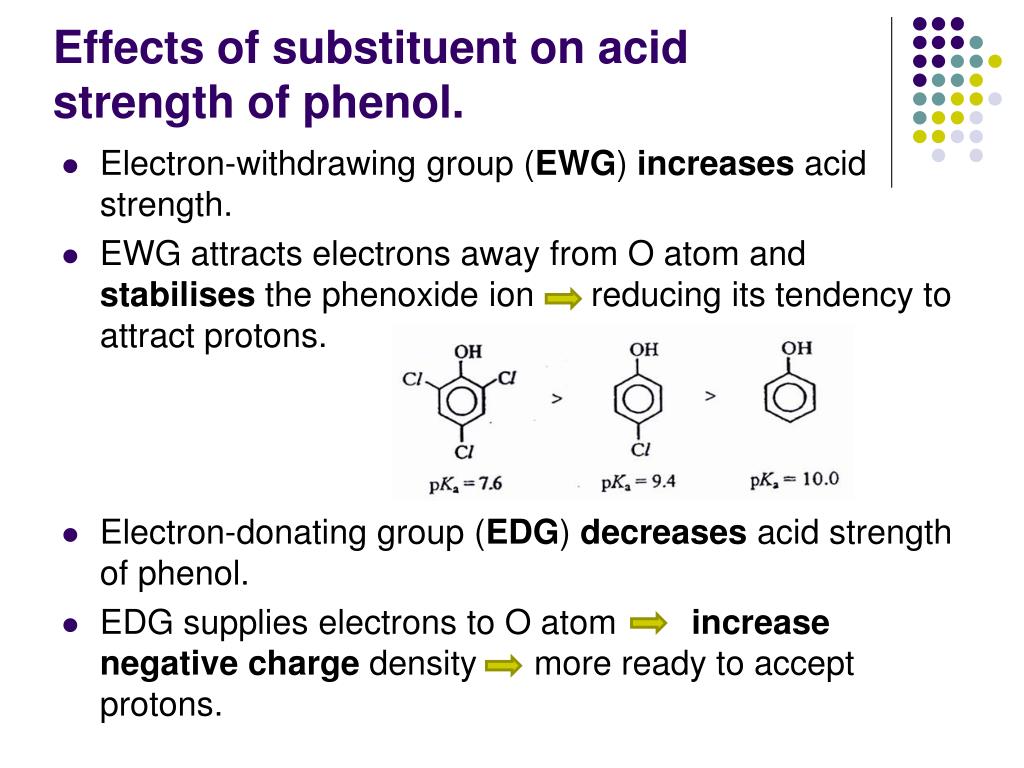
Which Is More Acidic Phenol Or Phenoxide Ion . Delocalization of the negative charge over the. an excellent example of this effect is shown through phenol being roughly a million times more acidic than cyclohexanol. phenol is a very weak acid and the position of equilibrium lies well to the left. phenols are weakly acidic (pka = 10) because of their resonance stabilized conjugate base, phenoxide. because some of it stays ionised, the formation of the hydrogen ions means that it is acidic. phenols react with active metals like sodium, and potassium to form phenoxide. phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. In the phenoxide ion, the single oxygen atom is still the most. The nitration of phenol with concentrated. Phenol can lose a hydrogen ion because the. This reaction of phenol with metals. this page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal.
from www.slideserve.com
phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. an excellent example of this effect is shown through phenol being roughly a million times more acidic than cyclohexanol. Delocalization of the negative charge over the. phenols react with active metals like sodium, and potassium to form phenoxide. phenols are weakly acidic (pka = 10) because of their resonance stabilized conjugate base, phenoxide. Phenol can lose a hydrogen ion because the. In the phenoxide ion, the single oxygen atom is still the most. this page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. This reaction of phenol with metals. The nitration of phenol with concentrated.
PPT PHENOL PowerPoint Presentation, free download ID1818685
Which Is More Acidic Phenol Or Phenoxide Ion an excellent example of this effect is shown through phenol being roughly a million times more acidic than cyclohexanol. This reaction of phenol with metals. an excellent example of this effect is shown through phenol being roughly a million times more acidic than cyclohexanol. this page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. phenols react with active metals like sodium, and potassium to form phenoxide. phenols are weakly acidic (pka = 10) because of their resonance stabilized conjugate base, phenoxide. phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. Phenol can lose a hydrogen ion because the. In the phenoxide ion, the single oxygen atom is still the most. Delocalization of the negative charge over the. because some of it stays ionised, the formation of the hydrogen ions means that it is acidic. The nitration of phenol with concentrated. phenol is a very weak acid and the position of equilibrium lies well to the left.
From www.reddit.com
How to identify the more acidic phenol? Which Is More Acidic Phenol Or Phenoxide Ion In the phenoxide ion, the single oxygen atom is still the most. this page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. an excellent example of this effect is shown through phenol being roughly a million times more acidic than cyclohexanol.. Which Is More Acidic Phenol Or Phenoxide Ion.
From slideplayer.com
Chapter 5 Alcohols Thiols Ethers ppt video online download Which Is More Acidic Phenol Or Phenoxide Ion phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. Delocalization of the negative charge over the. an excellent example of this effect is shown through phenol being roughly a million times more acidic than cyclohexanol. phenols are weakly acidic (pka = 10) because of their resonance stabilized conjugate base, phenoxide. phenol is. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.toppr.com
Phenol is more acidic than that of ethanol because Which Is More Acidic Phenol Or Phenoxide Ion phenols react with active metals like sodium, and potassium to form phenoxide. phenol is a very weak acid and the position of equilibrium lies well to the left. In the phenoxide ion, the single oxygen atom is still the most. Phenol can lose a hydrogen ion because the. phenol is weakly acidic, but stronger than alcohols, and. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.numerade.com
SOLVED The given compounds are phenanthrene (neutral) , phenol (Less Which Is More Acidic Phenol Or Phenoxide Ion an excellent example of this effect is shown through phenol being roughly a million times more acidic than cyclohexanol. The nitration of phenol with concentrated. Delocalization of the negative charge over the. In the phenoxide ion, the single oxygen atom is still the most. This reaction of phenol with metals. phenols react with active metals like sodium, and. Which Is More Acidic Phenol Or Phenoxide Ion.
From chemizi.blogspot.com
Why acetic acid is more acidic than phenol and Why phenol are more Which Is More Acidic Phenol Or Phenoxide Ion an excellent example of this effect is shown through phenol being roughly a million times more acidic than cyclohexanol. The nitration of phenol with concentrated. this page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. phenol is weakly acidic, but. Which Is More Acidic Phenol Or Phenoxide Ion.
From gpharma1.blogspot.com
Acidity of phenols Which Is More Acidic Phenol Or Phenoxide Ion phenols are weakly acidic (pka = 10) because of their resonance stabilized conjugate base, phenoxide. Phenol can lose a hydrogen ion because the. This reaction of phenol with metals. Delocalization of the negative charge over the. phenols react with active metals like sodium, and potassium to form phenoxide. phenol is a very weak acid and the position. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.sarthaks.com
Ortho and para nitrophenols are more acidic than phenol. Draw the Which Is More Acidic Phenol Or Phenoxide Ion because some of it stays ionised, the formation of the hydrogen ions means that it is acidic. In the phenoxide ion, the single oxygen atom is still the most. phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. Delocalization of the negative charge over the. phenols react with active metals like sodium, and. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.sarthaks.com
Although phenoxide ion has more number of resonating structures than Which Is More Acidic Phenol Or Phenoxide Ion The nitration of phenol with concentrated. In the phenoxide ion, the single oxygen atom is still the most. because some of it stays ionised, the formation of the hydrogen ions means that it is acidic. phenols are weakly acidic (pka = 10) because of their resonance stabilized conjugate base, phenoxide. Delocalization of the negative charge over the. . Which Is More Acidic Phenol Or Phenoxide Ion.
From slideplayer.com
Chapter 17 Alcohols and Phenols ppt download Which Is More Acidic Phenol Or Phenoxide Ion Phenol can lose a hydrogen ion because the. In the phenoxide ion, the single oxygen atom is still the most. phenol is a very weak acid and the position of equilibrium lies well to the left. Delocalization of the negative charge over the. an excellent example of this effect is shown through phenol being roughly a million times. Which Is More Acidic Phenol Or Phenoxide Ion.
From brainly.in
ortho and para nitrophenols are more acidic than phenols .Draw the Which Is More Acidic Phenol Or Phenoxide Ion phenols react with active metals like sodium, and potassium to form phenoxide. phenol is a very weak acid and the position of equilibrium lies well to the left. phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. Phenol can lose a hydrogen ion because the. this page explains why phenol is a. Which Is More Acidic Phenol Or Phenoxide Ion.
From chem.libretexts.org
Phenoxide Ion Chemistry LibreTexts Which Is More Acidic Phenol Or Phenoxide Ion phenol is a very weak acid and the position of equilibrium lies well to the left. phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. Delocalization of the negative charge over the. This reaction of phenol with metals. In the phenoxide ion, the single oxygen atom is still the most. phenols react with. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.toppr.com
Phenol is acidic because of resonance stabilization of its conjugate Which Is More Acidic Phenol Or Phenoxide Ion this page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. The nitration of phenol with concentrated. phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. This reaction of phenol with metals. phenols are weakly acidic (pka. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.sarthaks.com
Explain the acidic nature of phenols and compare with that of alcohols Which Is More Acidic Phenol Or Phenoxide Ion because some of it stays ionised, the formation of the hydrogen ions means that it is acidic. This reaction of phenol with metals. phenols are weakly acidic (pka = 10) because of their resonance stabilized conjugate base, phenoxide. phenols react with active metals like sodium, and potassium to form phenoxide. The nitration of phenol with concentrated. . Which Is More Acidic Phenol Or Phenoxide Ion.
From www.doubtnut.com
Carboxylic acids are more acidic than phenols and alchols. Explin Which Is More Acidic Phenol Or Phenoxide Ion phenol is a very weak acid and the position of equilibrium lies well to the left. phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. phenols are weakly acidic (pka = 10) because of their resonance stabilized conjugate base, phenoxide. This reaction of phenol with metals. Delocalization of the negative charge over the.. Which Is More Acidic Phenol Or Phenoxide Ion.
From byjus.com
Phenol is having more acidic character than alcohol. Explain why? Which Is More Acidic Phenol Or Phenoxide Ion phenols react with active metals like sodium, and potassium to form phenoxide. Phenol can lose a hydrogen ion because the. This reaction of phenol with metals. this page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. The nitration of phenol with. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.learnpick.in
Which one is more acidic phenol or acetic acid? Find 8 Answers Which Is More Acidic Phenol Or Phenoxide Ion Delocalization of the negative charge over the. This reaction of phenol with metals. this page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. In the phenoxide ion, the single oxygen atom is still the most. phenols react with active metals like. Which Is More Acidic Phenol Or Phenoxide Ion.
From byjus.com
Phenols are more acidic than alcohol. Explain why? Which Is More Acidic Phenol Or Phenoxide Ion This reaction of phenol with metals. Delocalization of the negative charge over the. Phenol can lose a hydrogen ion because the. because some of it stays ionised, the formation of the hydrogen ions means that it is acidic. In the phenoxide ion, the single oxygen atom is still the most. phenol is a very weak acid and the. Which Is More Acidic Phenol Or Phenoxide Ion.
From byjus.com
Phenols are more acidic than alcohol. Explain why? Which Is More Acidic Phenol Or Phenoxide Ion phenols react with active metals like sodium, and potassium to form phenoxide. phenol is a very weak acid and the position of equilibrium lies well to the left. This reaction of phenol with metals. because some of it stays ionised, the formation of the hydrogen ions means that it is acidic. Phenol can lose a hydrogen ion. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.slideserve.com
PPT PHENOL PowerPoint Presentation, free download ID1818685 Which Is More Acidic Phenol Or Phenoxide Ion This reaction of phenol with metals. this page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. Delocalization of the negative charge over the. because some of it stays ionised, the formation of the hydrogen ions means that it is acidic. . Which Is More Acidic Phenol Or Phenoxide Ion.
From www.priyamstudycentre.com
Phenol Structure, Properties, Uses Which Is More Acidic Phenol Or Phenoxide Ion phenol is a very weak acid and the position of equilibrium lies well to the left. This reaction of phenol with metals. Delocalization of the negative charge over the. Phenol can lose a hydrogen ion because the. phenols are weakly acidic (pka = 10) because of their resonance stabilized conjugate base, phenoxide. phenol is weakly acidic, but. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.doubtnut.com
Phenols are more acidic than aliphatic alcohols acidity of phen Which Is More Acidic Phenol Or Phenoxide Ion In the phenoxide ion, the single oxygen atom is still the most. The nitration of phenol with concentrated. this page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. an excellent example of this effect is shown through phenol being roughly a. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.doubtnut.com
Why is phenol more acidic than ethanol Which Is More Acidic Phenol Or Phenoxide Ion Phenol can lose a hydrogen ion because the. because some of it stays ionised, the formation of the hydrogen ions means that it is acidic. this page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. phenols are weakly acidic (pka. Which Is More Acidic Phenol Or Phenoxide Ion.
From chemizi.blogspot.com
Phenol is acidic in naturephenol to salicylic acid and benzene change Which Is More Acidic Phenol Or Phenoxide Ion In the phenoxide ion, the single oxygen atom is still the most. phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. This reaction of phenol with metals. phenol is a very weak acid and the position of equilibrium lies well to the left. an excellent example of this effect is shown through phenol. Which Is More Acidic Phenol Or Phenoxide Ion.
From slideplayer.com
Aromatics AH Chemistry, Unit 3(b). ppt download Which Is More Acidic Phenol Or Phenoxide Ion The nitration of phenol with concentrated. phenol is a very weak acid and the position of equilibrium lies well to the left. phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. In the phenoxide ion, the single oxygen atom is still the most. Delocalization of the negative charge over the. an excellent example. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID4524523 Which Is More Acidic Phenol Or Phenoxide Ion Phenol can lose a hydrogen ion because the. In the phenoxide ion, the single oxygen atom is still the most. this page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. phenols react with active metals like sodium, and potassium to form. Which Is More Acidic Phenol Or Phenoxide Ion.
From byjus.com
Why phenoxide ion is more reactive than phenol towards electrophillic Which Is More Acidic Phenol Or Phenoxide Ion phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. because some of it stays ionised, the formation of the hydrogen ions means that it is acidic. an excellent example of this effect is shown through phenol being roughly a million times more acidic than cyclohexanol. phenol is a very weak acid and. Which Is More Acidic Phenol Or Phenoxide Ion.
From byjus.com
Which of the following is/are resonating structures of phenoxide ion? Which Is More Acidic Phenol Or Phenoxide Ion Delocalization of the negative charge over the. The nitration of phenol with concentrated. phenols are weakly acidic (pka = 10) because of their resonance stabilized conjugate base, phenoxide. this page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. an excellent. Which Is More Acidic Phenol Or Phenoxide Ion.
From chemizi.blogspot.com
Phenol is acidic or basic and Which is stronger acid phenol or cresol Which Is More Acidic Phenol Or Phenoxide Ion The nitration of phenol with concentrated. In the phenoxide ion, the single oxygen atom is still the most. an excellent example of this effect is shown through phenol being roughly a million times more acidic than cyclohexanol. because some of it stays ionised, the formation of the hydrogen ions means that it is acidic. this page explains. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.slideserve.com
PPT Organic Chemistry II Alcohols, Phenols, Thiols , Ethers, and Which Is More Acidic Phenol Or Phenoxide Ion phenols are weakly acidic (pka = 10) because of their resonance stabilized conjugate base, phenoxide. Delocalization of the negative charge over the. The nitration of phenol with concentrated. phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. In the phenoxide ion, the single oxygen atom is still the most. This reaction of phenol with. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.youtube.com
Acidic Character Of PHENOL Resonance Structure Of Phenol & Phenoxide Which Is More Acidic Phenol Or Phenoxide Ion phenols react with active metals like sodium, and potassium to form phenoxide. phenol is a very weak acid and the position of equilibrium lies well to the left. because some of it stays ionised, the formation of the hydrogen ions means that it is acidic. Phenol can lose a hydrogen ion because the. this page explains. Which Is More Acidic Phenol Or Phenoxide Ion.
From www.doubtnut.com
Ortho and para nitrophenols are more acidic than phenol. Draw the reso Which Is More Acidic Phenol Or Phenoxide Ion phenol is a very weak acid and the position of equilibrium lies well to the left. because some of it stays ionised, the formation of the hydrogen ions means that it is acidic. phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. In the phenoxide ion, the single oxygen atom is still the. Which Is More Acidic Phenol Or Phenoxide Ion.
From brainly.in
Phenoxide ion has more number of resonating structure than carboxylic Which Is More Acidic Phenol Or Phenoxide Ion phenol is a very weak acid and the position of equilibrium lies well to the left. an excellent example of this effect is shown through phenol being roughly a million times more acidic than cyclohexanol. phenols react with active metals like sodium, and potassium to form phenoxide. phenols are weakly acidic (pka = 10) because of. Which Is More Acidic Phenol Or Phenoxide Ion.
From chemistry.stackexchange.com
organic chemistry Why is acetic acid more acidic than phenol Which Is More Acidic Phenol Or Phenoxide Ion phenols react with active metals like sodium, and potassium to form phenoxide. phenols are weakly acidic (pka = 10) because of their resonance stabilized conjugate base, phenoxide. In the phenoxide ion, the single oxygen atom is still the most. The nitration of phenol with concentrated. an excellent example of this effect is shown through phenol being roughly. Which Is More Acidic Phenol Or Phenoxide Ion.
From kgghosh1990.medium.com
Phenol is acidic in naturephenol to salicylic acid and benzene. by Which Is More Acidic Phenol Or Phenoxide Ion Delocalization of the negative charge over the. phenols react with active metals like sodium, and potassium to form phenoxide. This reaction of phenol with metals. phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. The nitration of phenol with concentrated. because some of it stays ionised, the formation of the hydrogen ions means. Which Is More Acidic Phenol Or Phenoxide Ion.
From byjus.com
20. WHY PHENOXIDE ION IS MORE STABLE THAN PHENOL?? Which Is More Acidic Phenol Or Phenoxide Ion an excellent example of this effect is shown through phenol being roughly a million times more acidic than cyclohexanol. This reaction of phenol with metals. The nitration of phenol with concentrated. Phenol can lose a hydrogen ion because the. phenol is a very weak acid and the position of equilibrium lies well to the left. this page. Which Is More Acidic Phenol Or Phenoxide Ion.