How To Prepare Salt Bridge . Click a picture below to view a larger version. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). It is a fairly simple device and can just be a string, or a piece of cotton that has been soaked in an. The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of ions or solute from. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. Alkali cations and halide anions would be ideal for this purpose. What would you put inside a salt bridge? Let's go through how to prepare an electrochemical salt bridge. The purpose of the salt bridge is to keep the. A salt bridge allows the flow of. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. First, it is important to put ionic species into the salt bridge that will not be reduced or oxidized in either of the half cells. A salt bridge is made from twisted string. The salt bridge is a vital component of any voltaic cell.
from waterpursuit.com
A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of ions or solute from. The purpose of the salt bridge is to keep the. A salt bridge is made from twisted string. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. What would you put inside a salt bridge? Click a picture below to view a larger version. The salt bridge is a vital component of any voltaic cell. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). A salt bridge allows the flow of.
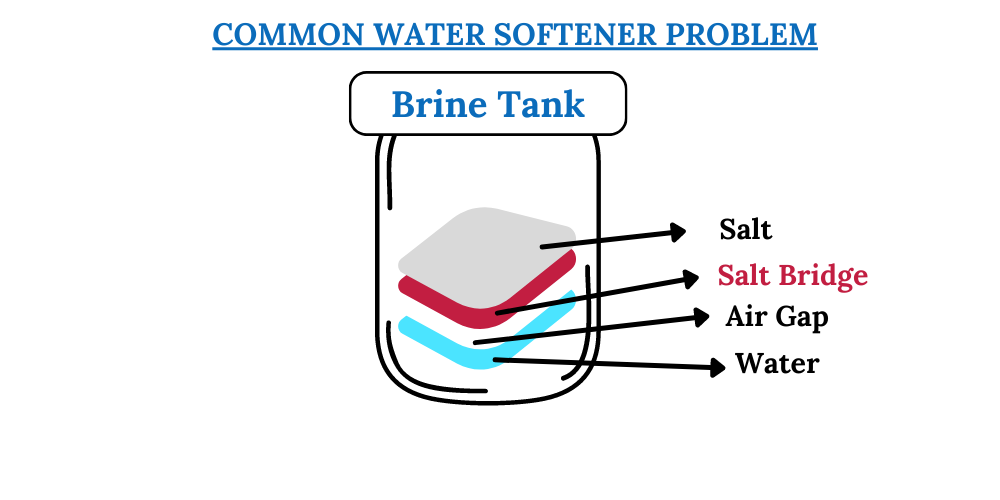
Potassium vs sodium water softener Which softener salt is better?
How To Prepare Salt Bridge A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. What would you put inside a salt bridge? A salt bridge is made from twisted string. First, it is important to put ionic species into the salt bridge that will not be reduced or oxidized in either of the half cells. The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of ions or solute from. Let's go through how to prepare an electrochemical salt bridge. Alkali cations and halide anions would be ideal for this purpose. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. The purpose of the salt bridge is to keep the. The salt bridge is a vital component of any voltaic cell. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. A salt bridge allows the flow of. Click a picture below to view a larger version. It is a fairly simple device and can just be a string, or a piece of cotton that has been soaked in an. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s).
From www.pinterest.com.mx
Salt Bridge Science student, Science rules, Science facts How To Prepare Salt Bridge Alkali cations and halide anions would be ideal for this purpose. Click a picture below to view a larger version. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). A salt bridge is made from twisted string. In other words, a salt bridge is a junction that connects the anodic and cathodic. How To Prepare Salt Bridge.
From achs-prod.acs.org
A Lateral Salt Bridge for the Specific Assembly of an ABCType Collagen How To Prepare Salt Bridge It is a fairly simple device and can just be a string, or a piece of cotton that has been soaked in an. First, it is important to put ionic species into the salt bridge that will not be reduced or oxidized in either of the half cells. What would you put inside a salt bridge? The salt bridge is. How To Prepare Salt Bridge.
From www.youtube.com
Functions of Salt bridge part 7 electro chemistry CBSE chemistry How To Prepare Salt Bridge First, it is important to put ionic species into the salt bridge that will not be reduced or oxidized in either of the half cells. Alkali cations and halide anions would be ideal for this purpose. The salt bridge is a vital component of any voltaic cell. It is a tube filled with an electrolyte solution such as kno 3. How To Prepare Salt Bridge.
From www.youtube.com
Salt bridge What is Salt Brigde Application of salt bridge How To Prepare Salt Bridge The salt bridge is a vital component of any voltaic cell. A salt bridge allows the flow of. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). First, it is important to put ionic species into the salt bridge that will not be reduced or oxidized in either of the half cells.. How To Prepare Salt Bridge.
From www.youtube.com
Salt bridge and it's functions.[video5] YouTube How To Prepare Salt Bridge It is a fairly simple device and can just be a string, or a piece of cotton that has been soaked in an. First, it is important to put ionic species into the salt bridge that will not be reduced or oxidized in either of the half cells. It is a tube filled with an electrolyte solution such as kno. How To Prepare Salt Bridge.
From www.vedantu.com
Electrochemistry Class 12 Notes for NEET Chemistry Revision How To Prepare Salt Bridge A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. What would you put inside a salt bridge? The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of ions or solute from. In. How To Prepare Salt Bridge.
From www.reddit.com
Galvanic cell salt bridges Mcat How To Prepare Salt Bridge The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of ions or solute from. What would you put inside a salt bridge? The salt bridge is a vital component of any voltaic cell. The purpose of the salt bridge is to keep the. In other words, a salt. How To Prepare Salt Bridge.
From www.youtube.com
Describe the function of a salt bridge. YouTube How To Prepare Salt Bridge The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of ions or solute from. Let's go through how to prepare an electrochemical salt bridge. Alkali cations and halide anions would be ideal for this purpose. A salt bridge allows the flow of. The purpose of the salt bridge. How To Prepare Salt Bridge.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID4831905 How To Prepare Salt Bridge The purpose of the salt bridge is to keep the. Alkali cations and halide anions would be ideal for this purpose. First, it is important to put ionic species into the salt bridge that will not be reduced or oxidized in either of the half cells. A salt bridge is made from twisted string. The salt bridge is a vital. How To Prepare Salt Bridge.
From byjus.com
Salt Bridge Definition, Function, Types, Preparation, Galvanic Cells How To Prepare Salt Bridge It is a fairly simple device and can just be a string, or a piece of cotton that has been soaked in an. The purpose of the salt bridge is to keep the. A salt bridge allows the flow of. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). Let's go through. How To Prepare Salt Bridge.
From www.youtube.com
Making a Salt Bridge 3 Methods YouTube How To Prepare Salt Bridge The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of ions or solute from. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The purpose of the salt bridge is to keep the. The salt bridge is a vital component. How To Prepare Salt Bridge.
From guidewiringkathmandu.z5.web.core.windows.net
Salt Bridge Diagram How To Prepare Salt Bridge A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. A salt bridge is made from twisted string. The salt bridge is a vital component of any voltaic cell. Click a picture below to view a larger version. Let's go through how to prepare an. How To Prepare Salt Bridge.
From www.youtube.com
5 Electrochemistry How Salt Bridge Works? Movement of ions in Salt How To Prepare Salt Bridge A salt bridge allows the flow of. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. Click a picture below to view. How To Prepare Salt Bridge.
From www.youtube.com
FUNCTION OF SALT BRIDGE YouTube How To Prepare Salt Bridge First, it is important to put ionic species into the salt bridge that will not be reduced or oxidized in either of the half cells. Let's go through how to prepare an electrochemical salt bridge. It is a fairly simple device and can just be a string, or a piece of cotton that has been soaked in an. In other. How To Prepare Salt Bridge.
From www.youtube.com
FUNCTION OF SALT BRIDGE (Chemistry Form 5) YouTube How To Prepare Salt Bridge In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. The purpose of the salt bridge is to keep the. What would you put inside a salt bridge? The salt bridge is a vital component of any voltaic cell. The purpose of an agar salt bridge is to. How To Prepare Salt Bridge.
From knoxvillewatertreatment.blogspot.com
Knoxville Water Treatment Salt Bridge DIY Repair How To Prepare Salt Bridge The purpose of the salt bridge is to keep the. The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of ions or solute from. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is. How To Prepare Salt Bridge.
From www.youtube.com
How the salt bridge works YouTube How To Prepare Salt Bridge The salt bridge is a vital component of any voltaic cell. A salt bridge allows the flow of. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. Alkali cations and halide anions would be ideal for this purpose. The purpose of an agar salt. How To Prepare Salt Bridge.
From www.youtube.com
Salt Bridge Function YouTube How To Prepare Salt Bridge Let's go through how to prepare an electrochemical salt bridge. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. It is a fairly simple device and can just be a string, or a piece of cotton that has been soaked in an. In other. How To Prepare Salt Bridge.
From www.youtube.com
Salt Bridge YouTube How To Prepare Salt Bridge What would you put inside a salt bridge? It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). Click a picture below to view a larger version. The purpose of the salt bridge is to keep the. The purpose of an agar salt bridge is to provide an electrical connection to the bath. How To Prepare Salt Bridge.
From waterfilterguru.com
What is a Water Softener Salt Bridge? (+How to Fix It) How To Prepare Salt Bridge First, it is important to put ionic species into the salt bridge that will not be reduced or oxidized in either of the half cells. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. It is a fairly simple device and can just be a string, or. How To Prepare Salt Bridge.
From www.youtube.com
How Salt bridges effect protein properties YouTube How To Prepare Salt Bridge A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. What would you put inside a salt bridge? The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of ions or solute from. It. How To Prepare Salt Bridge.
From www.youtube.com
Salt bridge (Electrochemistry part 9 for CBSE class 12 JEE IIT) YouTube How To Prepare Salt Bridge The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of ions or solute from. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. Click a picture below to view a larger version. A salt bridge. How To Prepare Salt Bridge.
From lavelle.chem.ucla.edu
Anode/Cathode/Salt Bridge daigram CHEMISTRY COMMUNITY How To Prepare Salt Bridge Alkali cations and halide anions would be ideal for this purpose. A salt bridge allows the flow of. The purpose of the salt bridge is to keep the. The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of ions or solute from. Let's go through how to prepare. How To Prepare Salt Bridge.
From waterpursuit.com
Potassium vs sodium water softener Which softener salt is better? How To Prepare Salt Bridge A salt bridge allows the flow of. The purpose of the salt bridge is to keep the. The salt bridge is a vital component of any voltaic cell. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). Alkali cations and halide anions would be ideal for this purpose. A salt bridge is. How To Prepare Salt Bridge.
From www.youtube.com
4 ELECTROCHEMISTRY SALT BRIDGE FUNCTION OF SALT BRIDGE IIT How To Prepare Salt Bridge Click a picture below to view a larger version. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. Let's go through how to prepare an electrochemical salt bridge. The purpose of an agar salt bridge is to provide an electrical connection to the bath. How To Prepare Salt Bridge.
From www.youtube.com
What is salt Bridge & state its function Electrochemistry Physical How To Prepare Salt Bridge It is a fairly simple device and can just be a string, or a piece of cotton that has been soaked in an. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. Let's go through how to prepare an electrochemical salt bridge. Alkali cations and halide anions. How To Prepare Salt Bridge.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID4491325 How To Prepare Salt Bridge The purpose of the salt bridge is to keep the. First, it is important to put ionic species into the salt bridge that will not be reduced or oxidized in either of the half cells. A salt bridge allows the flow of. A salt bridge is made from twisted string. What would you put inside a salt bridge? A salt. How To Prepare Salt Bridge.
From www.pineresearch.com
Reference Electrode Salt Bridge Kit Pine Research Instrumentation Store How To Prepare Salt Bridge What would you put inside a salt bridge? A salt bridge is made from twisted string. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of. How To Prepare Salt Bridge.
From chemistry.stackexchange.com
chemistry Why the change of the position of the salt bridge How To Prepare Salt Bridge Click a picture below to view a larger version. It is a fairly simple device and can just be a string, or a piece of cotton that has been soaked in an. The purpose of the salt bridge is to keep the. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half. How To Prepare Salt Bridge.
From www.researchgate.net
174 questions with answers in ELECTROANALYTICAL CHEMISTRY Scientific How To Prepare Salt Bridge Let's go through how to prepare an electrochemical salt bridge. The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of ions or solute from. In other words, a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. It is. How To Prepare Salt Bridge.
From www.youtube.com
Making a Ushaped Tube for a Salt Bridge YouTube How To Prepare Salt Bridge A salt bridge is made from twisted string. It is a fairly simple device and can just be a string, or a piece of cotton that has been soaked in an. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The salt bridge is a vital component of any voltaic cell. A. How To Prepare Salt Bridge.
From www.youtube.com
Salt bridge YouTube How To Prepare Salt Bridge It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). A salt bridge allows the flow of. Click a picture below to view a larger version. The salt bridge is a vital component of any voltaic cell. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and. How To Prepare Salt Bridge.
From www.youtube.com
Tutorial for Salt Bridge making YouTube How To Prepare Salt Bridge Alkali cations and halide anions would be ideal for this purpose. A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. What would you put inside a salt bridge? A salt bridge is made from twisted string. The purpose of the salt bridge is to. How To Prepare Salt Bridge.
From www.youtube.com
Salt Bridge YouTube How To Prepare Salt Bridge What would you put inside a salt bridge? A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). A salt bridge is made from twisted string. In other. How To Prepare Salt Bridge.
From www.youtube.com
Salt bridges in proteins YouTube How To Prepare Salt Bridge The salt bridge is a vital component of any voltaic cell. The purpose of an agar salt bridge is to provide an electrical connection to the bath solution while minimizing the transfer of ions or solute from. A salt bridge allows the flow of. It is a fairly simple device and can just be a string, or a piece of. How To Prepare Salt Bridge.