What Is The Osmolarity Of Water . Osmolality is the concentration of osmoles in a. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. While any polar solvent could be used, these. It is defined as the number of. Osmolarity is nearly the same as osmolality. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. An osmole is 1 mole of any fully dissociated substance dissolved in water. Find the osmolarity of nacl by multiplying the value in moles by 2. Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre.
from www.youtube.com
An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity is nearly the same as osmolality. Osmolality is the concentration of osmoles in a. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. While any polar solvent could be used, these. Find the osmolarity of nacl by multiplying the value in moles by 2. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology.
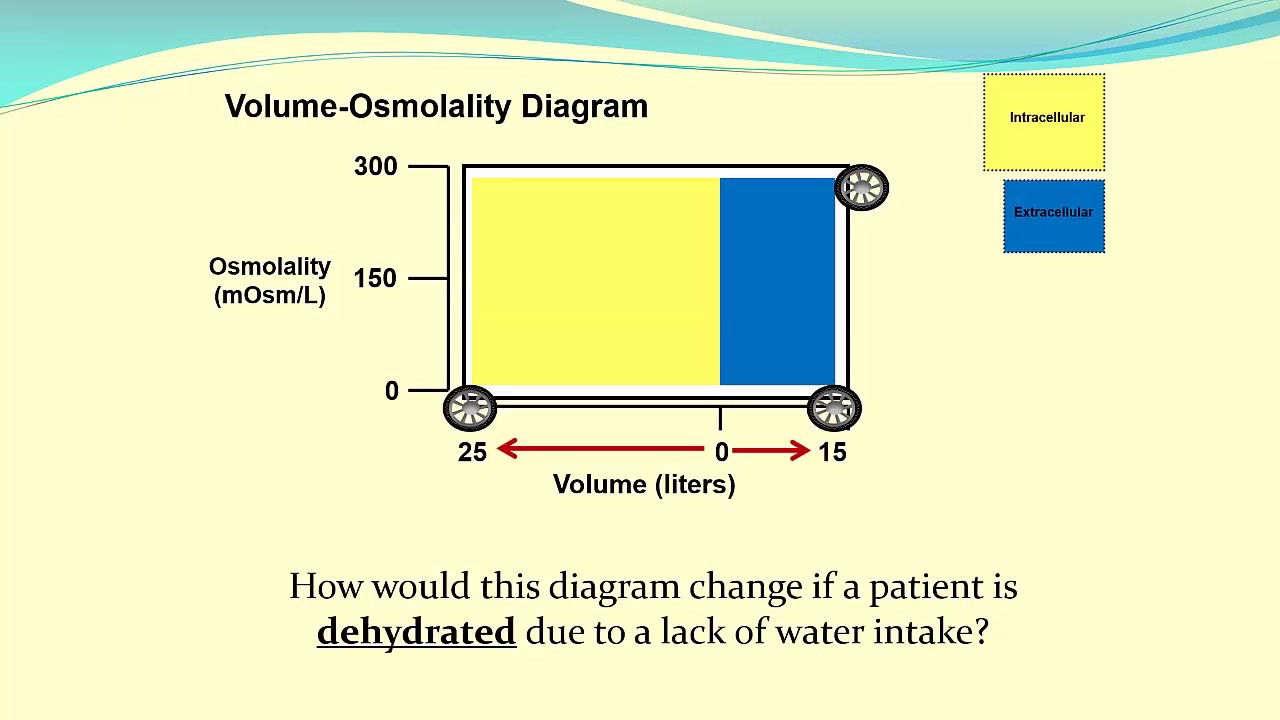
Using VolumeOsmolality Diagrams to Understand Body Fluid Status YouTube
What Is The Osmolarity Of Water Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. An osmole is 1 mole of any fully dissociated substance dissolved in water. While any polar solvent could be used, these. Find the osmolarity of nacl by multiplying the value in moles by 2. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. Osmolality is the concentration of osmoles in a. Osmolarity is nearly the same as osmolality. It is defined as the number of.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube What Is The Osmolarity Of Water Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity is nearly the same as osmolality. Find the osmolarity of nacl by multiplying the value in moles by 2. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. It is defined as the number of. An osmole. What Is The Osmolarity Of Water.
From drawittoknowit.com
Anatomy & Physiology Osmosis and Osmolarity ditki medical What Is The Osmolarity Of Water Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. It is defined as the number of. Find the osmolarity of nacl by multiplying the value in moles by 2. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolality is the concentration of. What Is The Osmolarity Of Water.
From droualb.faculty.mjc.edu
Chapter 4 What Is The Osmolarity Of Water Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. It is defined as the number of. Osmolality is the concentration of osmoles in a. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmolarity is. What Is The Osmolarity Of Water.
From www.slideshare.net
Lecture 4 What Is The Osmolarity Of Water Osmolality is a measure of the concentration of particles in the serum per kilogram of water. While any polar solvent could be used, these. Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Find the osmolarity of nacl by multiplying the value in moles by 2. It is defined as the number. What Is The Osmolarity Of Water.
From biologydictionary.net
Osmolarity The Definitive Guide Biology Dictionary What Is The Osmolarity Of Water Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. While any polar solvent could be used, these. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmolarity is nearly the. What Is The Osmolarity Of Water.
From www.youtube.com
Osmolarity Example (Bio) YouTube What Is The Osmolarity Of Water Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. It is defined as the number of. Find the osmolarity of nacl by multiplying the value in moles by 2. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. While any polar solvent could be used, these.. What Is The Osmolarity Of Water.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download What Is The Osmolarity Of Water Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Osmolarity is nearly the same as osmolality. Find the osmolarity of nacl by multiplying the value in moles by 2. An osmole is 1 mole of any fully dissociated substance dissolved in water. It is defined as the number of. Osmotic. What Is The Osmolarity Of Water.
From www.slideserve.com
PPT Body Fluids PowerPoint Presentation, free download ID2152192 What Is The Osmolarity Of Water Osmolality is the concentration of osmoles in a. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. It is defined as the number of. Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Osmolarity and osmolality are units of solute concentration that are often used in. What Is The Osmolarity Of Water.
From www.slideshare.net
Regulation of osmolarity What Is The Osmolarity Of Water Osmolarity is nearly the same as osmolality. Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Osmolality is the concentration of osmoles in a.. What Is The Osmolarity Of Water.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 2. Osmolarity ditki What Is The Osmolarity Of Water Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity is nearly the same as osmolality. An osmole is 1 mole of any. What Is The Osmolarity Of Water.
From www.chegg.com
Solved What is the osmolarity in mOsm/L of a solution of What Is The Osmolarity Of Water Osmolality is the concentration of osmoles in a. An osmole is 1 mole of any fully dissociated substance dissolved in water. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. While any polar solvent could be used, these. It. What Is The Osmolarity Of Water.
From www.slideserve.com
PPT Body Fluids PowerPoint Presentation, free download ID2087885 What Is The Osmolarity Of Water Find the osmolarity of nacl by multiplying the value in moles by 2. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and. What Is The Osmolarity Of Water.
From www.youtube.com
Osmolality vs Osmolarity (with a mnemonic) Physiology and Chemistry What Is The Osmolarity Of Water Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. It is defined as the number of. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Learn the. What Is The Osmolarity Of Water.
From www.coastalwiki.org
Osmosis Coastal Wiki What Is The Osmolarity Of Water Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Find the osmolarity of nacl by multiplying the value in moles by 2. Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Osmolarity is nearly the same as osmolality. It is defined as the number of. Osmolarity. What Is The Osmolarity Of Water.
From drawittoknowit.com
Biochemistry Glossary Osmosis & Osmolarity 1. Osmosis Draw It to What Is The Osmolarity Of Water Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Osmolarity is nearly the same as osmolality. Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Osmotic concentration,. What Is The Osmolarity Of Water.
From www.slideserve.com
PPT BODY FLUID COMPARTMENT AND FLUID BALANCE PowerPoint Presentation What Is The Osmolarity Of Water Find the osmolarity of nacl by multiplying the value in moles by 2. Osmolarity is nearly the same as osmolality. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. It is defined as the number of. Osmolality is the. What Is The Osmolarity Of Water.
From quizlet.com
changes in osmolarity of tubular fluid Diagram Quizlet What Is The Osmolarity Of Water Osmolarity is nearly the same as osmolality. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolality is the concentration of osmoles in a. Osmolarity and osmolality are units of solute concentration that are often used in reference. What Is The Osmolarity Of Water.
From www.slideserve.com
PPT Osmoregulation and Excretion PowerPoint Presentation, free What Is The Osmolarity Of Water Osmolarity is nearly the same as osmolality. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Find the osmolarity of nacl by multiplying the value in moles by 2. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. While any polar solvent could. What Is The Osmolarity Of Water.
From www.slideserve.com
PPT Osmoregulation = keeping water and salt balanced in the body What Is The Osmolarity Of Water Osmolality is the concentration of osmoles in a. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Osmolarity is nearly the same as. What Is The Osmolarity Of Water.
From pediaa.com
Difference Between Osmolarity and Osmolality Definition, Explanation What Is The Osmolarity Of Water Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Osmolality is the concentration of osmoles in a. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. An osmole is 1 mole of any fully dissociated substance dissolved in water. While any polar. What Is The Osmolarity Of Water.
From www.researchgate.net
Comparison of the osmolarity of ambient seawater and lobster hemolymph What Is The Osmolarity Of Water Find the osmolarity of nacl by multiplying the value in moles by 2. It is defined as the number of. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. Osmotic concentration, formerly known as osmolarity, is the measure. What Is The Osmolarity Of Water.
From ibiologia.com
Osmolarity Definition, Formula & Osmolarity vs. Osmolality What Is The Osmolarity Of Water While any polar solvent could be used, these. Find the osmolarity of nacl by multiplying the value in moles by 2. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Osmotic concentration, formerly known. What Is The Osmolarity Of Water.
From www.slideserve.com
PPT Homeostasis Osmoregulation in elasmobranchs PowerPoint What Is The Osmolarity Of Water Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Osmolarity is nearly the same as osmolality. It is defined as the number of. While any polar solvent could be used, these. Learn the. What Is The Osmolarity Of Water.
From www.slideserve.com
PPT Osmoregulation PowerPoint Presentation, free download ID4770499 What Is The Osmolarity Of Water Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Find the osmolarity of nacl by multiplying the value in moles by 2. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Osmolality is the concentration of osmoles in a. Osmolality is a measure of. What Is The Osmolarity Of Water.
From www.slideserve.com
PPT Fluid & Electrolyte Balance PowerPoint Presentation, free What Is The Osmolarity Of Water Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. While any polar solvent could be used, these. Find the osmolarity of nacl by multiplying the value in moles by 2. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. It is defined as the number. What Is The Osmolarity Of Water.
From www.slideserve.com
PPT Water Metabolism PowerPoint Presentation, free download ID4493121 What Is The Osmolarity Of Water Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. Osmolarity and osmolality are units of solute concentration that are often used in. What Is The Osmolarity Of Water.
From www.youtube.com
How to solve osmolarity calculation problems YouTube What Is The Osmolarity Of Water Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Osmolarity is nearly the same as osmolality. Find. What Is The Osmolarity Of Water.
From www.slideshare.net
7Membrane Transport I What Is The Osmolarity Of Water Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Osmotic concentration, formerly known as osmolarity, is the. What Is The Osmolarity Of Water.
From www.youtube.com
Using VolumeOsmolality Diagrams to Understand Body Fluid Status YouTube What Is The Osmolarity Of Water Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. Osmolarity and osmolality are units of solute concentration that are often used in. What Is The Osmolarity Of Water.
From www.numerade.com
SOLVED What is the osmolarity of a 0.179 M aqueous potassium carbonate What Is The Osmolarity Of Water Osmolarity is nearly the same as osmolality. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. It is defined as the number of. Osmolality is the concentration of osmoles in a. Learn the definitions and. What Is The Osmolarity Of Water.
From www.numerade.com
SOLVED 15. Calculate the osmolarity of a solution containing 45.00 g What Is The Osmolarity Of Water Osmolarity is nearly the same as osmolality. While any polar solvent could be used, these. Find the osmolarity of nacl by multiplying the value in moles by 2. Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution.. What Is The Osmolarity Of Water.
From www.goconqr.com
Regulation of Water Balance ( Osmolarity ) Mind Map What Is The Osmolarity Of Water Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids. Osmolality is the concentration of osmoles in a. While any polar solvent could be used, these. Osmolarity is nearly the same as osmolality. It is defined as the number of. Osmolality is a measure of the concentration of particles in the. What Is The Osmolarity Of Water.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download What Is The Osmolarity Of Water While any polar solvent could be used, these. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. Osmolality is the concentration of osmoles in a. Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration in a solution. Find the osmolarity of nacl by multiplying the value in moles by 2. An. What Is The Osmolarity Of Water.
From biologydictionary.net
Osmolarity The Definitive Guide Biology Dictionary What Is The Osmolarity Of Water Osmolality is the concentration of osmoles in a. Osmolarity is nearly the same as osmolality. It is defined as the number of. An osmole is 1 mole of any fully dissociated substance dissolved in water. Find the osmolarity of nacl by multiplying the value in moles by 2. Osmolarity and osmolality are units of solute concentration that are often used. What Is The Osmolarity Of Water.
From lookfordiagnosis.com
Osmolar Concentration; Ionic Strength; Osmolarity; Osmolality What Is The Osmolarity Of Water Osmolality is the number of particles per kilogram of liquid, while osmolarity is the number per litre. Osmolality is the concentration of osmoles in a. Osmolarity is nearly the same as osmolality. Learn the definitions and concepts of osmolarity, tonicity, osmolality and osmotic pressure in fluid physiology. Osmolarity and osmolality are units of solute concentration that are often used in. What Is The Osmolarity Of Water.