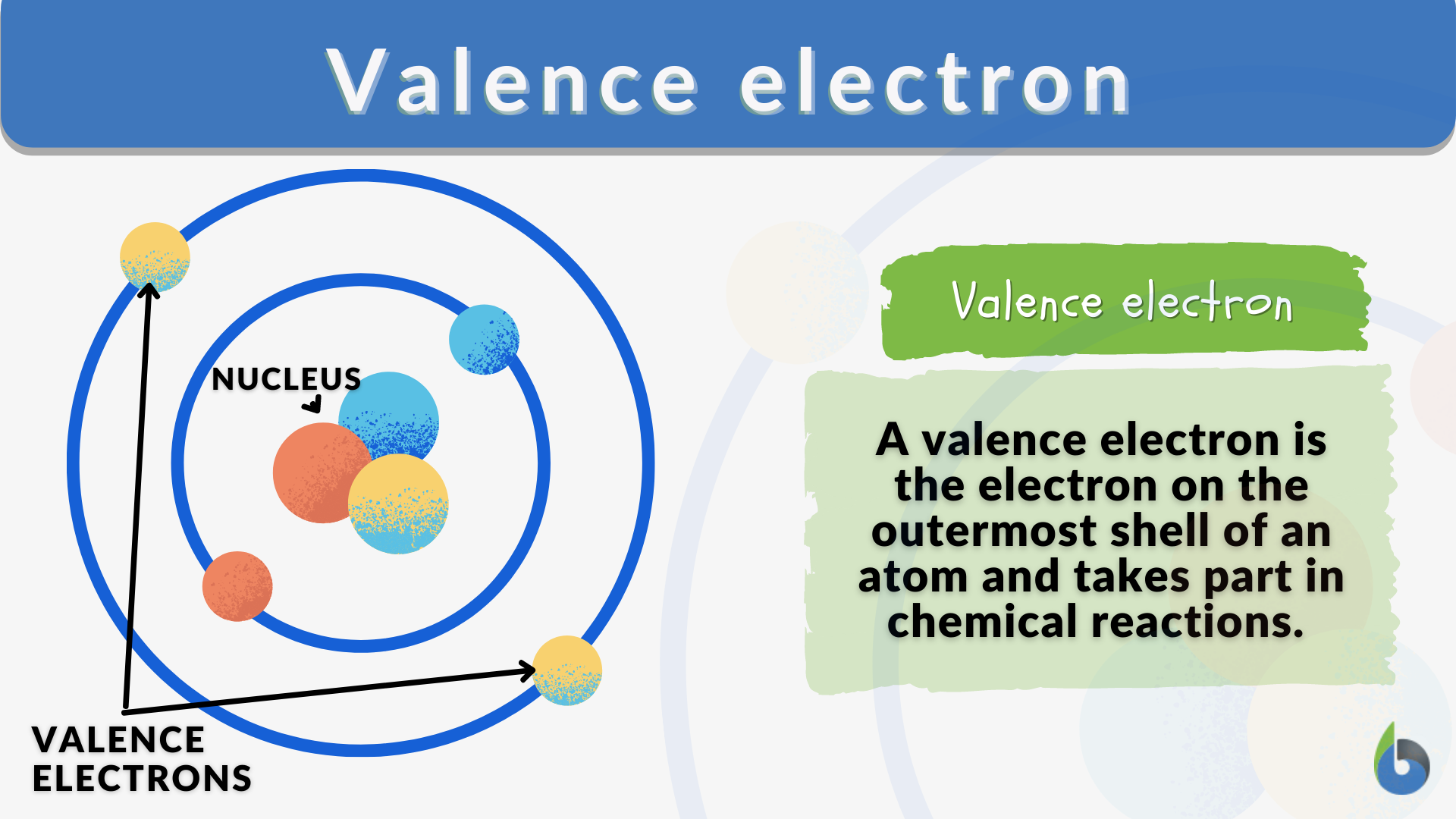
What Is The Number Of Valence Electrons In N And F . For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. both n and p are in group 15 and have five valence electrons. the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. This is why elements in the same group have similar chemical. valence electrons are the electrons in the outermost shell, or. number of valence electrons. The number of electrons in an atom's outermost valence shell governs its bonding behaviour. in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. 119 rows atomic number elements valence electrons;
from www.biologyonline.com
both n and p are in group 15 and have five valence electrons. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. number of valence electrons. The number of electrons in an atom's outermost valence shell governs its bonding behaviour. This is why elements in the same group have similar chemical. the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. valence electrons are the electrons in the outermost shell, or. 119 rows atomic number elements valence electrons; in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached.
Valence electron Definition and Examples Biology Online Dictionary
What Is The Number Of Valence Electrons In N And F This is why elements in the same group have similar chemical. number of valence electrons. The number of electrons in an atom's outermost valence shell governs its bonding behaviour. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. valence electrons are the electrons in the outermost shell, or. This is why elements in the same group have similar chemical. 119 rows atomic number elements valence electrons; in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. both n and p are in group 15 and have five valence electrons.
From www.youtube.com
Valence and core electrons YouTube What Is The Number Of Valence Electrons In N And F the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. both n and p are in group 15 and have five valence electrons. 119 rows atomic number elements valence electrons; This is why elements in the same group have similar chemical. For main group elements,. What Is The Number Of Valence Electrons In N And F.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is The Number Of Valence Electrons In N And F number of valence electrons. valence electrons are the electrons in the outermost shell, or. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. This is why elements in the same group have similar chemical. in fact, the number of valence electrons goes up by one for each step. What Is The Number Of Valence Electrons In N And F.
From www.revimage.org
General Valence Electron Ground State Configuration For Neutral What Is The Number Of Valence Electrons In N And F the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. 119 rows atomic number elements valence electrons; The number of electrons in an atom's outermost valence shell governs its bonding behaviour. both n and p are in group 15 and have five valence electrons. . What Is The Number Of Valence Electrons In N And F.
From www.ck12.org
The Periodic Table and Electron Configurations CK12 Foundation What Is The Number Of Valence Electrons In N And F valence electrons are the electrons in the outermost shell, or. 119 rows atomic number elements valence electrons; in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight. What Is The Number Of Valence Electrons In N And F.
From iperiodictable.com
Oxygen Valence Electrons (O) Oxygen Valency & Electron Configuration What Is The Number Of Valence Electrons In N And F This is why elements in the same group have similar chemical. the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. 119 rows atomic number elements valence electrons;. What Is The Number Of Valence Electrons In N And F.
From jamaicahomebuilders.blogspot.com
Number Of Valence Electrons Definition / Difference Between Valency and What Is The Number Of Valence Electrons In N And F number of valence electrons. 119 rows atomic number elements valence electrons; valence electrons are the electrons in the outermost shell, or. The number of electrons in an atom's outermost valence shell governs its bonding behaviour. both n and p are in group 15 and have five valence electrons. in fact, the number of valence electrons. What Is The Number Of Valence Electrons In N And F.
From www.slideshare.net
Valence Electrons What Is The Number Of Valence Electrons In N And F For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. This is why elements in the same group have similar chemical. valence electrons are the electrons in. What Is The Number Of Valence Electrons In N And F.
From www.biologyonline.com
Valence electron Definition and Examples Biology Online Dictionary What Is The Number Of Valence Electrons In N And F in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. valence electrons are the electrons in the outermost shell, or. The number of electrons in an atom's outermost valence shell governs its bonding behaviour. number of valence electrons. the number of valence electrons. What Is The Number Of Valence Electrons In N And F.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is The Number Of Valence Electrons In N And F the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. This is why elements in the same group have similar chemical. number. What Is The Number Of Valence Electrons In N And F.
From www.vrogue.co
What Are Valence Electrons Definition And Periodic Ta vrogue.co What Is The Number Of Valence Electrons In N And F valence electrons are the electrons in the outermost shell, or. in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. both n and p are in group 15 and have five valence electrons. 119 rows atomic number elements valence electrons; number of. What Is The Number Of Valence Electrons In N And F.
From valenceelectrons.com
How to Find the Valence Electrons for ClO2 and ClO2? What Is The Number Of Valence Electrons In N And F number of valence electrons. This is why elements in the same group have similar chemical. valence electrons are the electrons in the outermost shell, or. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. the number of valence electrons in one atom of each element is easily determined. What Is The Number Of Valence Electrons In N And F.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is The Number Of Valence Electrons In N And F the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. This is why elements in the same group have similar chemical. number of valence electrons. both n and p are in group 15 and have five valence electrons. valence electrons are the electrons in. What Is The Number Of Valence Electrons In N And F.
From www.numerade.com
SOLVEDDetermine the number of valence electrons in an atom of each of What Is The Number Of Valence Electrons In N And F both n and p are in group 15 and have five valence electrons. This is why elements in the same group have similar chemical. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. valence electrons are the electrons in the outermost shell, or. The number of electrons in an. What Is The Number Of Valence Electrons In N And F.
From thebiglebowski566.blogspot.com
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio What Is The Number Of Valence Electrons In N And F valence electrons are the electrons in the outermost shell, or. both n and p are in group 15 and have five valence electrons. the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. 119 rows atomic number elements valence electrons; number of valence. What Is The Number Of Valence Electrons In N And F.
From ellisrestroulner94.blogspot.com
Silicon Electron Configuration How Many Unpaired Electrons Ellis What Is The Number Of Valence Electrons In N And F number of valence electrons. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. valence electrons are the electrons in the outermost shell, or. in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. 119. What Is The Number Of Valence Electrons In N And F.
From christoperroth.blogspot.com
what are valence electrons definition and periodic table chapter 2 What Is The Number Of Valence Electrons In N And F This is why elements in the same group have similar chemical. The number of electrons in an atom's outermost valence shell governs its bonding behaviour. the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. 119 rows atomic number elements valence electrons; For main group elements,. What Is The Number Of Valence Electrons In N And F.
From selftution.com
Valency and Variable Valency Valence Shell and Electrons » Selftution What Is The Number Of Valence Electrons In N And F 119 rows atomic number elements valence electrons; number of valence electrons. The number of electrons in an atom's outermost valence shell governs its bonding behaviour. This is why elements in the same group have similar chemical. the number of valence electrons in one atom of each element is easily determined based on its position in the periodic. What Is The Number Of Valence Electrons In N And F.
From www.youtube.com
Electron arrangement & valence electrons YouTube What Is The Number Of Valence Electrons In N And F This is why elements in the same group have similar chemical. the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. The number of electrons in an atom's outermost valence shell governs its bonding behaviour. number of valence electrons. both n and p are in. What Is The Number Of Valence Electrons In N And F.
From chem.libretexts.org
6.9 Electron Configurations and the Periodic Table Chemistry LibreTexts What Is The Number Of Valence Electrons In N And F valence electrons are the electrons in the outermost shell, or. The number of electrons in an atom's outermost valence shell governs its bonding behaviour. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. number of valence electrons. This is why elements in the same group have similar chemical. . What Is The Number Of Valence Electrons In N And F.
From www.youtube.com
Valence Electrons & Electron Configurations YouTube What Is The Number Of Valence Electrons In N And F in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. number of valence electrons. the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. valence electrons are the electrons in the. What Is The Number Of Valence Electrons In N And F.
From jamaicahomebuilders.blogspot.com
Number Of Valence Electrons Definition / Difference Between Valency and What Is The Number Of Valence Electrons In N And F For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. This is why elements in the same group have similar chemical. in fact, the number of valence electrons. What Is The Number Of Valence Electrons In N And F.
From valenceelectrons.com
Fluorine Electron Configuration With Full Orbital Diagram What Is The Number Of Valence Electrons In N And F both n and p are in group 15 and have five valence electrons. The number of electrons in an atom's outermost valence shell governs its bonding behaviour. number of valence electrons. the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. For main group elements,. What Is The Number Of Valence Electrons In N And F.
From topblogtenz.com
How to find Valence electrons Various method and Examples What Is The Number Of Valence Electrons In N And F valence electrons are the electrons in the outermost shell, or. The number of electrons in an atom's outermost valence shell governs its bonding behaviour. 119 rows atomic number elements valence electrons; both n and p are in group 15 and have five valence electrons. number of valence electrons. the number of valence electrons in one. What Is The Number Of Valence Electrons In N And F.
From valenceelectrons.com
How to Find the Valence Electrons for Titanium (Ti)? What Is The Number Of Valence Electrons In N And F number of valence electrons. 119 rows atomic number elements valence electrons; The number of electrons in an atom's outermost valence shell governs its bonding behaviour. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. the number of valence electrons in one atom of each element is easily determined. What Is The Number Of Valence Electrons In N And F.
From uhighlsu.web.fc2.com
valence electrons of silicon What Is The Number Of Valence Electrons In N And F The number of electrons in an atom's outermost valence shell governs its bonding behaviour. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. 119 rows atomic number elements valence electrons; number of valence electrons. the number of valence electrons in one atom of each element is easily determined. What Is The Number Of Valence Electrons In N And F.
From www.sliderbase.com
Valence Electrons Presentation Chemistry What Is The Number Of Valence Electrons In N And F The number of electrons in an atom's outermost valence shell governs its bonding behaviour. This is why elements in the same group have similar chemical. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. both n and p are in group 15 and have five valence electrons. 119 rows. What Is The Number Of Valence Electrons In N And F.
From whatsinsight.org
What is the number of valence electrons in nitrogen? What's Insight What Is The Number Of Valence Electrons In N And F in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. 119 rows atomic number elements valence electrons; The number of electrons in an atom's outermost valence shell governs its bonding behaviour. For main group elements, the number of valence electrons usually ranges between 1 and. What Is The Number Of Valence Electrons In N And F.
From wisc.pb.unizin.org
Core and Valence Electrons, Shielding, Zeff (M7Q8) UWMadison What Is The Number Of Valence Electrons In N And F valence electrons are the electrons in the outermost shell, or. 119 rows atomic number elements valence electrons; in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. The number of electrons in an atom's outermost valence shell governs its bonding behaviour. the number. What Is The Number Of Valence Electrons In N And F.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind What Is The Number Of Valence Electrons In N And F 119 rows atomic number elements valence electrons; both n and p are in group 15 and have five valence electrons. This is why elements in the same group have similar chemical. The number of electrons in an atom's outermost valence shell governs its bonding behaviour. valence electrons are the electrons in the outermost shell, or. the. What Is The Number Of Valence Electrons In N And F.
From www.physicsclassroom.com
Lewis Electron Dot Structures Counting Valence Electrons What Is The Number Of Valence Electrons In N And F For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. valence electrons are the electrons in the outermost shell, or. This is why elements in the same group have similar chemical. the number of valence electrons in one atom of each element is easily determined based on its position in. What Is The Number Of Valence Electrons In N And F.
From tonydavilio.com
How to find Valency? What are valence electrons? Teachoo (2023) What Is The Number Of Valence Electrons In N And F the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. number of valence electrons. 119 rows atomic number elements valence electrons; For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. in fact, the number of. What Is The Number Of Valence Electrons In N And F.
From www.vrogue.co
What Are Valence Electrons vrogue.co What Is The Number Of Valence Electrons In N And F the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. number of valence electrons. valence electrons are the electrons in the outermost shell, or. This is why elements in the same group have similar chemical. For main group elements, the number of valence electrons usually. What Is The Number Of Valence Electrons In N And F.
From www.pinterest.com.au
Valence Electrons Chart Chemistry Pinterest Chart, Chemistry and What Is The Number Of Valence Electrons In N And F in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. For main group elements, the number of valence electrons usually ranges between 1. What Is The Number Of Valence Electrons In N And F.
From www.periodictableprintable.com
How To Find Number Of Valence Electrons On Periodic Table Periodic What Is The Number Of Valence Electrons In N And F The number of electrons in an atom's outermost valence shell governs its bonding behaviour. in fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. For main group elements, the number of valence electrons usually ranges between 1 and 8 because eight electrons. the number of. What Is The Number Of Valence Electrons In N And F.
From santiagomeowmeyer.blogspot.com
Why Are Only Valence Electrons Involved in Bonding What Is The Number Of Valence Electrons In N And F the number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. 119 rows atomic number elements valence electrons; both n and p are in group 15 and have five valence electrons. For main group elements, the number of valence electrons usually ranges between 1 and 8. What Is The Number Of Valence Electrons In N And F.