Why Is Distilled Water Boiled Before Using It As A Ph Solution . It becomes distilled after boiling, making it pure h2o without contamination. To ensure the quality and consistency of boiled water, it is recommended to: The reason that distilled water is boiled prior to use in preparing titration solutions is to remove dissolved co2 which is present in. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. When water is filtered to remove different types of impurities, it becomes distilled. Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect its acidity or. An accurate determination of ph should made from a sample of distilled and cooled water, i.e. The ph of distilled water. It's highly volatile and fragile. At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. A solution with a ph of 7 at. Use filtered or distilled water:
from waterfiltersadvisor.com
The reason that distilled water is boiled prior to use in preparing titration solutions is to remove dissolved co2 which is present in. Use filtered or distilled water: A solution with a ph of 7 at. An accurate determination of ph should made from a sample of distilled and cooled water, i.e. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect its acidity or. To ensure the quality and consistency of boiled water, it is recommended to: At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. When water is filtered to remove different types of impurities, it becomes distilled. The ph of distilled water.
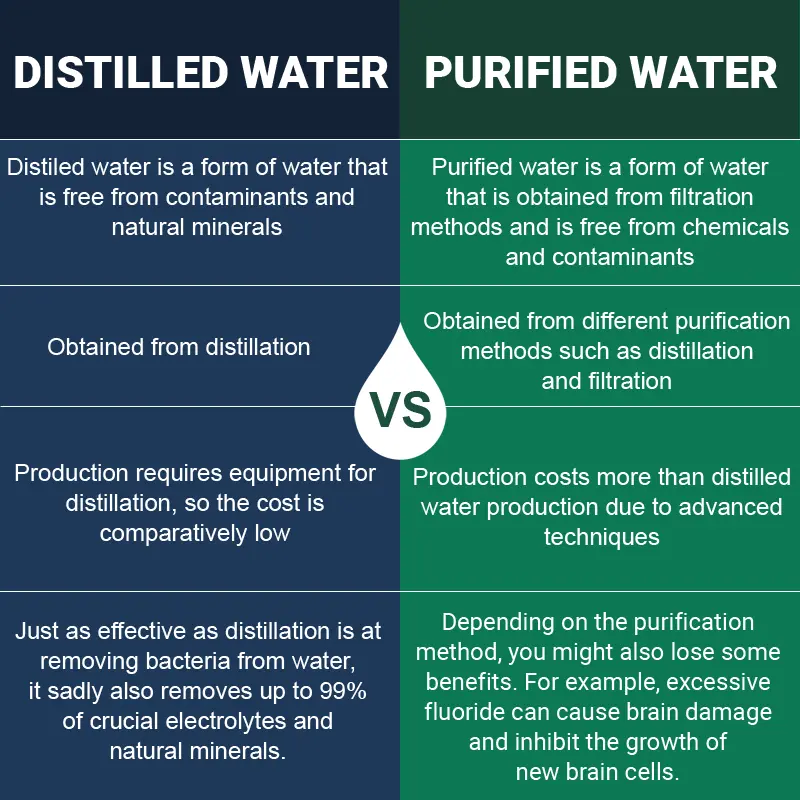
Distilled Vs. Purified Water (We Explain The Differences
Why Is Distilled Water Boiled Before Using It As A Ph Solution A solution with a ph of 7 at. An accurate determination of ph should made from a sample of distilled and cooled water, i.e. At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. To ensure the quality and consistency of boiled water, it is recommended to: A solution with a ph of 7 at. The reason that distilled water is boiled prior to use in preparing titration solutions is to remove dissolved co2 which is present in. When water is filtered to remove different types of impurities, it becomes distilled. It becomes distilled after boiling, making it pure h2o without contamination. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. The ph of distilled water. Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect its acidity or. Use filtered or distilled water: It's highly volatile and fragile.
From www.youtube.com
how to prepare distilled water distilled water preparation in Why Is Distilled Water Boiled Before Using It As A Ph Solution It becomes distilled after boiling, making it pure h2o without contamination. Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect its acidity or. The reason that distilled water is boiled prior to use in preparing titration solutions is to remove dissolved co2 which is present in. A solution with. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From watertechadvice.com
Deionized Water vs Distilled Here's the Main Differences Why Is Distilled Water Boiled Before Using It As A Ph Solution At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. When water is filtered to remove different types of impurities, it becomes distilled. It's highly volatile and fragile. A solution with a ph of 7 at. It becomes distilled after boiling, making it pure h2o without contamination. An accurate determination. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.distilledwater.co.uk
Where can I buy Distilled Water in the UK Distilled Water with Fast Why Is Distilled Water Boiled Before Using It As A Ph Solution Use filtered or distilled water: It's highly volatile and fragile. A solution with a ph of 7 at. An accurate determination of ph should made from a sample of distilled and cooled water, i.e. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. At 100°c, the ph of pure water is 6.14, which is neutral. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.watersmartsystems.com
Distilled Water vs Water Softener vs Filtered Water Detailed Why Is Distilled Water Boiled Before Using It As A Ph Solution The reason that distilled water is boiled prior to use in preparing titration solutions is to remove dissolved co2 which is present in. Use filtered or distilled water: At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. Distilled water, by nature, has a neutral ph of 7, but the. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From netsolwater.com
Is it safe to drink distilled water everyday? Distilled water Netsol Why Is Distilled Water Boiled Before Using It As A Ph Solution Use filtered or distilled water: To ensure the quality and consistency of boiled water, it is recommended to: An accurate determination of ph should made from a sample of distilled and cooled water, i.e. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. A solution with a ph of 7 at. It becomes distilled after. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From 5differencebetween.com
5 Difference Between Distilled and Purified Water Distilled vs Why Is Distilled Water Boiled Before Using It As A Ph Solution It becomes distilled after boiling, making it pure h2o without contamination. An accurate determination of ph should made from a sample of distilled and cooled water, i.e. A solution with a ph of 7 at. At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. Natural water has a ph. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From waterseer.org
Distilled Water Vs Deionized Water Know The Real Difference Why Is Distilled Water Boiled Before Using It As A Ph Solution An accurate determination of ph should made from a sample of distilled and cooled water, i.e. To ensure the quality and consistency of boiled water, it is recommended to: At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. It becomes distilled after boiling, making it pure h2o without contamination.. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.vevor.com
What is the pH of Distilled Water and Why Does It Matter? VEVOR Blog Why Is Distilled Water Boiled Before Using It As A Ph Solution When water is filtered to remove different types of impurities, it becomes distilled. The ph of distilled water. It's highly volatile and fragile. It becomes distilled after boiling, making it pure h2o without contamination. Use filtered or distilled water: The reason that distilled water is boiled prior to use in preparing titration solutions is to remove dissolved co2 which is. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From waterseer.org
Distilled Water VS Purified Water Side Effects, Uses, And More Why Is Distilled Water Boiled Before Using It As A Ph Solution Use filtered or distilled water: It's highly volatile and fragile. A solution with a ph of 7 at. At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. The ph of distilled water. When water is. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.sciencekiddo.com
Red Cabbage pH Indicator Kitchen Chemistry for Kids Science Kiddo Why Is Distilled Water Boiled Before Using It As A Ph Solution A solution with a ph of 7 at. It becomes distilled after boiling, making it pure h2o without contamination. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. When water is filtered to remove different types of impurities, it becomes distilled. Use filtered or distilled water: The ph of distilled water. At 100°c, the ph. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From humidifiersource.com
Why You Should Be Using Distilled Water In Your Humidifier Why Is Distilled Water Boiled Before Using It As A Ph Solution An accurate determination of ph should made from a sample of distilled and cooled water, i.e. Use filtered or distilled water: To ensure the quality and consistency of boiled water, it is recommended to: A solution with a ph of 7 at. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. At 100°c, the ph. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.smacgigworld.com
Difference Between Distilled Water and Deionized Water SMACgig World Why Is Distilled Water Boiled Before Using It As A Ph Solution It becomes distilled after boiling, making it pure h2o without contamination. To ensure the quality and consistency of boiled water, it is recommended to: Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. A solution with a ph of 7 at. The ph of distilled water. It's highly volatile and fragile. The reason that distilled. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.numerade.com
SOLVED Procedure HzO Part I Standardization of NaOH Hp Boil Why Is Distilled Water Boiled Before Using It As A Ph Solution When water is filtered to remove different types of impurities, it becomes distilled. The reason that distilled water is boiled prior to use in preparing titration solutions is to remove dissolved co2 which is present in. A solution with a ph of 7 at. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. At 100°c,. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.numerade.com
SOLVED Standardization of NaOH Prepare stock solution of NaOH using Why Is Distilled Water Boiled Before Using It As A Ph Solution It becomes distilled after boiling, making it pure h2o without contamination. An accurate determination of ph should made from a sample of distilled and cooled water, i.e. To ensure the quality and consistency of boiled water, it is recommended to: Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. It's highly volatile and fragile. Distilled. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.drlamcoaching.com
Distilled Water Is Purer, But Is It Really Better For Your Health? Why Is Distilled Water Boiled Before Using It As A Ph Solution The reason that distilled water is boiled prior to use in preparing titration solutions is to remove dissolved co2 which is present in. A solution with a ph of 7 at. When water is filtered to remove different types of impurities, it becomes distilled. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. At 100°c,. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From drinkprime.in
Distilled vs Purified Water Pro ,Cons and Comparison Why Is Distilled Water Boiled Before Using It As A Ph Solution It's highly volatile and fragile. To ensure the quality and consistency of boiled water, it is recommended to: At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. It becomes distilled after boiling, making it pure h2o without contamination. When water is filtered to remove different types of impurities, it. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From waterfiltersadvisor.com
Distilled Vs. Purified Water (We Explain The Differences Why Is Distilled Water Boiled Before Using It As A Ph Solution Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. To ensure the quality and consistency of boiled water, it is recommended to: It becomes distilled after boiling, making it pure h2o without contamination. The reason that distilled water is boiled prior to use in preparing titration solutions is to remove dissolved co2 which is present. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From slideplayer.com
Lab Activity 1 Active Acidity, pH, and Buffer ppt download Why Is Distilled Water Boiled Before Using It As A Ph Solution An accurate determination of ph should made from a sample of distilled and cooled water, i.e. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect its acidity or. When water is filtered to remove different. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.chemicals.co.uk
What’s the Difference Between Distilled & Ultrapure Water? The Why Is Distilled Water Boiled Before Using It As A Ph Solution Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect its acidity or. A solution with a ph of 7 at. The reason that distilled water is boiled prior to use in preparing titration solutions is. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.waterev.com
What is the PH of Distilled Water What is the PH value of water and Why Is Distilled Water Boiled Before Using It As A Ph Solution The ph of distilled water. When water is filtered to remove different types of impurities, it becomes distilled. Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect its acidity or. To ensure the quality and consistency of boiled water, it is recommended to: Natural water has a ph level. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.lifeionizers.com
Alkaline Water vs. Distilled Water which is healthier? Life Ionizers Why Is Distilled Water Boiled Before Using It As A Ph Solution It becomes distilled after boiling, making it pure h2o without contamination. It's highly volatile and fragile. The ph of distilled water. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. An accurate determination of ph should made from a sample of distilled and cooled water, i.e. To ensure the quality and consistency of boiled water,. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.youtube.com
Distention Between Distilled Water and Boiled Water YouTube Why Is Distilled Water Boiled Before Using It As A Ph Solution Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect its acidity or. The reason that distilled water is boiled prior to use in preparing titration solutions is to remove dissolved co2 which is present in. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline.. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.vevor.com
What is the pH of Distilled Water and Why Does It Matter? VEVOR Blog Why Is Distilled Water Boiled Before Using It As A Ph Solution It's highly volatile and fragile. To ensure the quality and consistency of boiled water, it is recommended to: Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect its acidity or. At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From netsolwater.com
How to Differentiate Between Boiled and Distilled Water Netsol Water Why Is Distilled Water Boiled Before Using It As A Ph Solution An accurate determination of ph should made from a sample of distilled and cooled water, i.e. To ensure the quality and consistency of boiled water, it is recommended to: It becomes distilled after boiling, making it pure h2o without contamination. Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From waterfilterportal.com
Distilled Water Vs Purified Water 2023 Ultimate Guide Why Is Distilled Water Boiled Before Using It As A Ph Solution At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. A solution with a ph of 7 at. Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect its acidity or. Use filtered or distilled water: When water is filtered. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From thewaterfiltermarket.com
Is Boiled Water the Same as Distilled Water? Water Filter Market Why Is Distilled Water Boiled Before Using It As A Ph Solution It's highly volatile and fragile. At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. A solution with a ph of 7 at. The ph of distilled water. Use filtered or distilled water: The reason that distilled water is boiled prior to use in preparing titration solutions is to remove. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.diffzy.com
Deionized vs. Distilled Water What's The Difference (With Table) Why Is Distilled Water Boiled Before Using It As A Ph Solution When water is filtered to remove different types of impurities, it becomes distilled. An accurate determination of ph should made from a sample of distilled and cooled water, i.e. At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. Use filtered or distilled water: Natural water has a ph level. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.pinterest.com
Is Boiled Water and Distilled Water The Same? (Not Exactly, Here’s Why Why Is Distilled Water Boiled Before Using It As A Ph Solution To ensure the quality and consistency of boiled water, it is recommended to: The reason that distilled water is boiled prior to use in preparing titration solutions is to remove dissolved co2 which is present in. Use filtered or distilled water: It's highly volatile and fragile. The ph of distilled water. It becomes distilled after boiling, making it pure h2o. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From quenchwater.com
Distilled Water vs Purified Water Quench Water Why Is Distilled Water Boiled Before Using It As A Ph Solution At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. Use filtered or distilled water: Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. When water is filtered to remove different types of impurities, it becomes distilled. To ensure the quality and consistency of boiled. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From pediaa.com
Difference Between Distilled Water and Purified Water Definition Why Is Distilled Water Boiled Before Using It As A Ph Solution When water is filtered to remove different types of impurities, it becomes distilled. It becomes distilled after boiling, making it pure h2o without contamination. Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect its acidity or. A solution with a ph of 7 at. Use filtered or distilled water:. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From askanydifference.com
Distilled Water vs Boiled Water Difference and Comparison Why Is Distilled Water Boiled Before Using It As A Ph Solution When water is filtered to remove different types of impurities, it becomes distilled. It's highly volatile and fragile. An accurate determination of ph should made from a sample of distilled and cooled water, i.e. A solution with a ph of 7 at. The ph of distilled water. Use filtered or distilled water: Distilled water, by nature, has a neutral ph. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From sciencetrends.com
What Is The pH Of Distilled Water? Science Trends Why Is Distilled Water Boiled Before Using It As A Ph Solution Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect its acidity or. The ph of distilled water. When water is filtered to remove different types of impurities, it becomes distilled. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. To ensure the quality and. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From www.chegg.com
Solved 1. Compare the pH values of boiled and unboiled Why Is Distilled Water Boiled Before Using It As A Ph Solution At 100°c, the ph of pure water is 6.14, which is neutral on the ph scale at this higher temperature. An accurate determination of ph should made from a sample of distilled and cooled water, i.e. The ph of distilled water. Natural water has a ph level of 6.9 to 7.5, which is slightly alkaline. Use filtered or distilled water:. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From humidifiersource.com
Distilled Water Vs. Boiled Water For Humidifier (The Answer May Why Is Distilled Water Boiled Before Using It As A Ph Solution The ph of distilled water. Distilled water, by nature, has a neutral ph of 7, but the process of boiling can introduce changes that affect its acidity or. An accurate determination of ph should made from a sample of distilled and cooled water, i.e. The reason that distilled water is boiled prior to use in preparing titration solutions is to. Why Is Distilled Water Boiled Before Using It As A Ph Solution.
From waterseer.org
Nursery Water Vs Distilled Water Which is Safe For Your Baby Why Is Distilled Water Boiled Before Using It As A Ph Solution It's highly volatile and fragile. It becomes distilled after boiling, making it pure h2o without contamination. An accurate determination of ph should made from a sample of distilled and cooled water, i.e. The reason that distilled water is boiled prior to use in preparing titration solutions is to remove dissolved co2 which is present in. Distilled water, by nature, has. Why Is Distilled Water Boiled Before Using It As A Ph Solution.