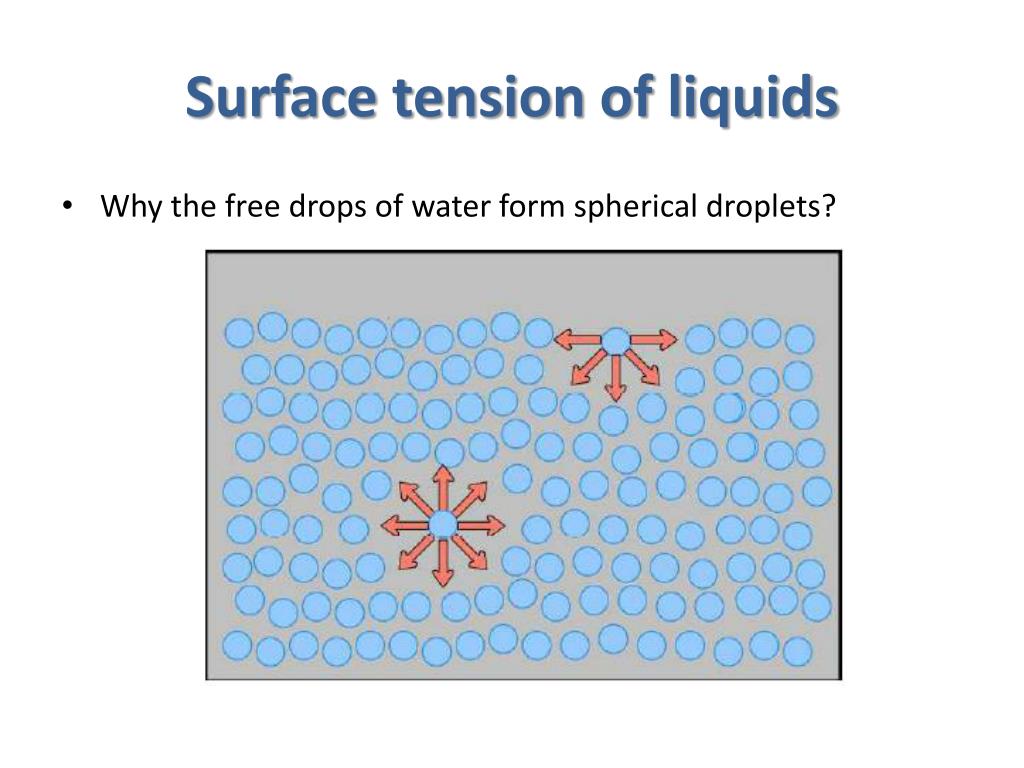
Surface Tension In Liquid . Since these intermolecular forces vary. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”.
from www.slideserve.com
Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Since these intermolecular forces vary. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free
Surface Tension In Liquid Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Since these intermolecular forces vary. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Surface Tension In Liquid Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. “surface tension is the tension of a. Surface Tension In Liquid.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Surface Tension In Liquid The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. Since these intermolecular forces vary. “surface tension is the tension of a liquid’s surface film. Surface Tension In Liquid.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Surface Tension In Liquid Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer,. Surface Tension In Liquid.
From www.slideserve.com
PPT LIQUID SURFACES PowerPoint Presentation, free download ID4456243 Surface Tension In Liquid Since these intermolecular forces vary. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is. Surface Tension In Liquid.
From ar.inspiredpencil.com
Surface Tension Water Surface Tension In Liquid Since these intermolecular forces vary. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in. Surface Tension In Liquid.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Surface Tension In Liquid Since these intermolecular forces vary. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. “surface tension is the tension of. Surface Tension In Liquid.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules Surface Tension In Liquid Since these intermolecular forces vary. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles. Surface Tension In Liquid.
From chem.libretexts.org
11.3 Some Properties of Liquids Chemistry LibreTexts Surface Tension In Liquid The molecules on the surface of a liquid are attracted by their neighbors from the. Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. “surface tension is the tension. Surface Tension In Liquid.
From www.youtube.com
Surface Tension of Water Explained YouTube Surface Tension In Liquid Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. Since these intermolecular forces vary. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The molecules on the surface of a liquid are. Surface Tension In Liquid.
From studyfullirene.z21.web.core.windows.net
Surface Tension Of Water Worksheet Surface Tension In Liquid Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest. Surface Tension In Liquid.
From johnjslatero.blob.core.windows.net
Surface Tension Of Water Def at johnjslatero blog Surface Tension In Liquid Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules on the surface of a. Surface Tension In Liquid.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID9168408 Surface Tension In Liquid “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Since these intermolecular forces vary. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. Surface tension is the energy, or work, required to. Surface Tension In Liquid.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension In Liquid Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which. Surface Tension In Liquid.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free Surface Tension In Liquid The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is a phenomenon that occurs due to the cohesive. Surface Tension In Liquid.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid Surface Tension In Liquid Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Since these intermolecular forces vary. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles. Surface Tension In Liquid.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids Surface Tension In Liquid Since these intermolecular forces vary. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the. Surface Tension In Liquid.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson Surface Tension In Liquid Since these intermolecular forces vary. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. “surface tension is. Surface Tension In Liquid.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 Surface Tension In Liquid Since these intermolecular forces vary. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of a liquid’s surface film caused by the bulk of the. Surface Tension In Liquid.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments Surface Tension In Liquid Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which. Surface Tension In Liquid.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free Surface Tension In Liquid Since these intermolecular forces vary. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. “surface tension is the tension of a. Surface Tension In Liquid.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical Surface Tension In Liquid The molecules on the surface of a liquid are attracted by their neighbors from the. Since these intermolecular forces vary. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the elastic tendency of. Surface Tension In Liquid.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Surface Tension In Liquid Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. The molecules on the surface of a liquid are attracted by their. Surface Tension In Liquid.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse Surface Tension In Liquid The molecules on the surface of a liquid are attracted by their neighbors from the. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid. Surface Tension In Liquid.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Surface Tension In Liquid Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is. Surface Tension In Liquid.
From www.biolinscientific.com
3 ways to measure surface tension Surface Tension In Liquid The molecules on the surface of a liquid are attracted by their neighbors from the. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is the energy, or work, required to increase the surface. Surface Tension In Liquid.
From www.thoughtco.com
Surface Tension Definition in Chemistry Surface Tension In Liquid The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Since these intermolecular forces vary. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Cohesive forces between molecules cause the. Surface Tension In Liquid.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii Surface Tension In Liquid Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension, property of a liquid surface displayed by its. Surface Tension In Liquid.
From www.thoughtco.com
Liquid Definition in Chemistry Surface Tension In Liquid Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension. Surface Tension In Liquid.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Surface Tension In Liquid Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension, property of a liquid surface. Surface Tension In Liquid.
From www.researchgate.net
2 Schematic representation of surface tension of a hemispherical water Surface Tension In Liquid Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface of a liquid are attracted by their neighbors from the. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer,. Surface Tension In Liquid.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Surface Tension In Liquid Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. “surface tension is the tension of a liquid’s. Surface Tension In Liquid.
From www.youtube.com
Surface Tension on Liquid Jet formula derivation with 3d animation Surface Tension In Liquid “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid. Surface Tension In Liquid.
From www.youtube.com
Surface Tension Super Ability Of Liquid Water on Penny Experiment Surface Tension In Liquid Since these intermolecular forces vary. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The molecules on the surface. Surface Tension In Liquid.
From www.biolinscientific.com
Surface tension of water Why is it so high? Surface Tension In Liquid Since these intermolecular forces vary. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the energy, or work, required to. Surface Tension In Liquid.
From www.writework.com
Surface Tension WriteWork Surface Tension In Liquid “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary. Cohesive forces between molecules. Surface Tension In Liquid.