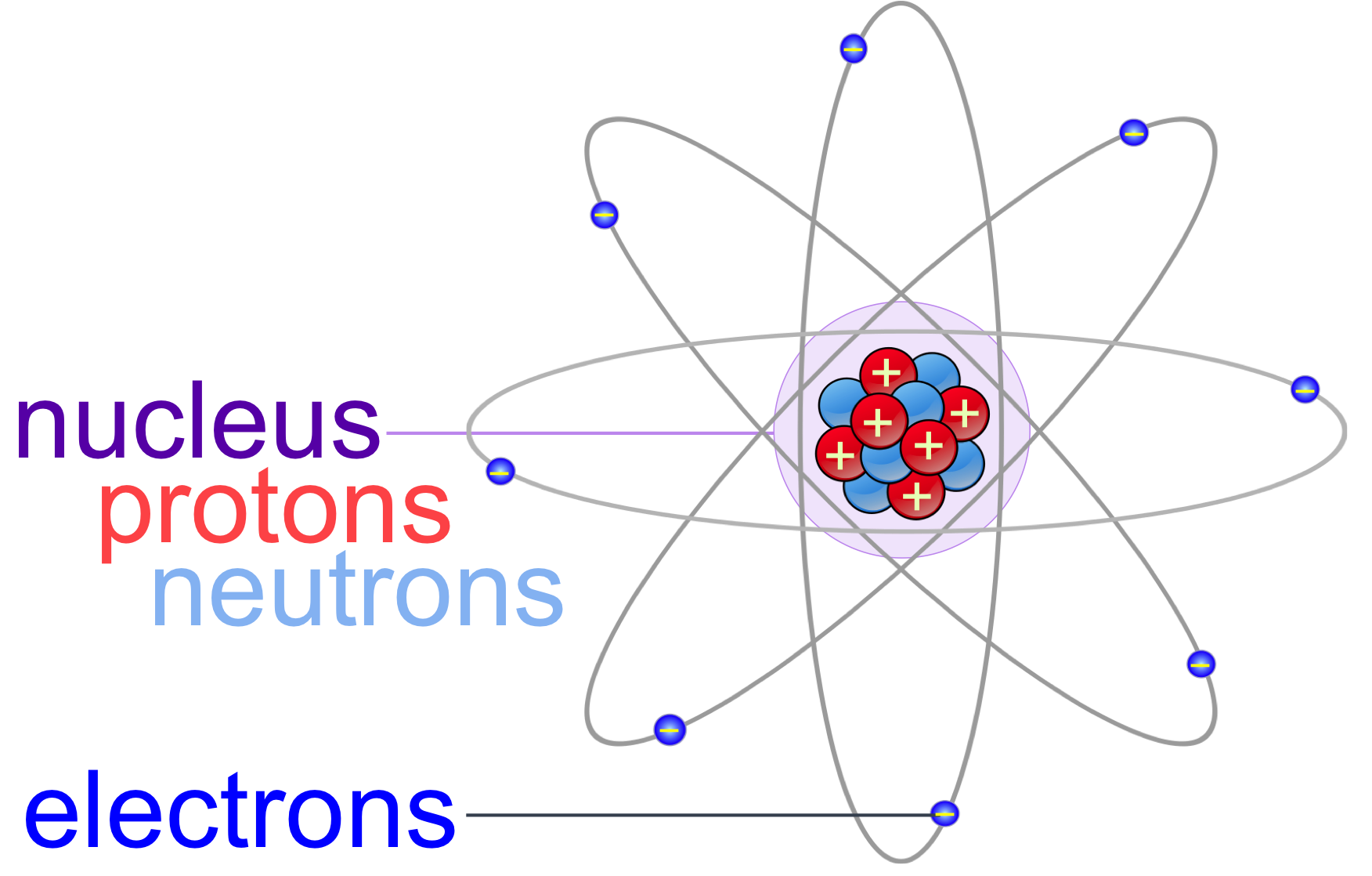
Do Positive Ions Have The Same Number Of Protons And Electrons . The atom is neutral as it has two positive protons and two negative electrons. In many cases, elements that. A neutral atom has the same number of protons and electrons. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. They contain the same number of protons as electrons. This number is the atomic number of the element. Metal atoms lose electrons from their outer shell when they form ions: A helium atom that has lost or gained an electron is an ion. A helium atom that has lost or gained an electron is an ion. The atom is neutral as it has two positive protons and two negative electrons. The ions are positive, because they have more. Recall that neutral atoms have the same number of protons and electrons. You can determine the number of electrons in an ion if you know. By definition, an ion is an electrically charged. In nature, however, many atoms lose or gain electrons.
from thebiologyprimer.com
The atom is neutral as it has two positive protons and two negative electrons. By definition, an ion is an electrically charged. In nature, however, many atoms lose or gain electrons. In many cases, elements that. The atom is neutral as it has two positive protons and two negative electrons. A helium atom that has lost or gained an electron is an ion. A neutral atom has the same number of protons and electrons. This number is the atomic number of the element. The ions are positive, because they have more. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge.
Atoms & Molecules echapter — The Biology Primer
Do Positive Ions Have The Same Number Of Protons And Electrons You can determine the number of electrons in an ion if you know. A helium atom that has lost or gained an electron is an ion. They contain the same number of protons as electrons. The atom is neutral as it has two positive protons and two negative electrons. This number is the atomic number of the element. A neutral atom has the same number of protons and electrons. The atom is neutral as it has two positive protons and two negative electrons. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. In nature, however, many atoms lose or gain electrons. Recall that neutral atoms have the same number of protons and electrons. Metal atoms lose electrons from their outer shell when they form ions: A helium atom that has lost or gained an electron is an ion. You can determine the number of electrons in an ion if you know. In many cases, elements that. The ions are positive, because they have more. By definition, an ion is an electrically charged.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Do Positive Ions Have The Same Number Of Protons And Electrons You can determine the number of electrons in an ion if you know. The atom is neutral as it has two positive protons and two negative electrons. This number is the atomic number of the element. In nature, however, many atoms lose or gain electrons. A neutral atom has the same number of protons and electrons. Metal atoms lose electrons. Do Positive Ions Have The Same Number Of Protons And Electrons.
From schematicmandorlas.z14.web.core.windows.net
Understanding Protons Electrons And Neutrons Do Positive Ions Have The Same Number Of Protons And Electrons The atom is neutral as it has two positive protons and two negative electrons. In many cases, elements that. The ions are positive, because they have more. You can determine the number of electrons in an ion if you know. Metal atoms lose electrons from their outer shell when they form ions: In nature, however, many atoms lose or gain. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.nagwa.com
Question Video Identifying What Changes When an Atom Forms a Positive Do Positive Ions Have The Same Number Of Protons And Electrons They contain the same number of protons as electrons. A helium atom that has lost or gained an electron is an ion. The atom is neutral as it has two positive protons and two negative electrons. Recall that neutral atoms have the same number of protons and electrons. By definition, an ion is an electrically charged. A neutral atom has. Do Positive Ions Have The Same Number Of Protons And Electrons.
From mungfali.com
Protons Neutrons And Electrons Diagram Do Positive Ions Have The Same Number Of Protons And Electrons This number is the atomic number of the element. They contain the same number of protons as electrons. The atom is neutral as it has two positive protons and two negative electrons. By definition, an ion is an electrically charged. In nature, however, many atoms lose or gain electrons. Often, the number of protons and electrons is not the same,. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.sliderbase.com
Formula of Ionic Compounds Do Positive Ions Have The Same Number Of Protons And Electrons They contain the same number of protons as electrons. You can determine the number of electrons in an ion if you know. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. In nature, however, many atoms lose or gain electrons. The atom is neutral as it has two. Do Positive Ions Have The Same Number Of Protons And Electrons.
From mybios.me
Using The Periodic Table To Determine Protons Neutrons And Electrons Do Positive Ions Have The Same Number Of Protons And Electrons They contain the same number of protons as electrons. In nature, however, many atoms lose or gain electrons. By definition, an ion is an electrically charged. The atom is neutral as it has two positive protons and two negative electrons. The ions are positive, because they have more. Often, the number of protons and electrons is not the same, so. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Do Positive Ions Have The Same Number Of Protons And Electrons A neutral atom has the same number of protons and electrons. Recall that neutral atoms have the same number of protons and electrons. By definition, an ion is an electrically charged. The ions are positive, because they have more. Metal atoms lose electrons from their outer shell when they form ions: The atom is neutral as it has two positive. Do Positive Ions Have The Same Number Of Protons And Electrons.
From utedzz.blogspot.com
Periodic Table Of Elements List With Protons Neutrons And Electrons Do Positive Ions Have The Same Number Of Protons And Electrons A helium atom that has lost or gained an electron is an ion. By definition, an ion is an electrically charged. They contain the same number of protons as electrons. You can determine the number of electrons in an ion if you know. In many cases, elements that. A helium atom that has lost or gained an electron is an. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.youtube.com
How To Calculate The Number of Protons, Neutrons, and Electrons Do Positive Ions Have The Same Number Of Protons And Electrons The ions are positive, because they have more. By definition, an ion is an electrically charged. A neutral atom has the same number of protons and electrons. Recall that neutral atoms have the same number of protons and electrons. You can determine the number of electrons in an ion if you know. The atom is neutral as it has two. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.examanalysis.in
Electric Current Do Positive Ions Have The Same Number Of Protons And Electrons Metal atoms lose electrons from their outer shell when they form ions: You can determine the number of electrons in an ion if you know. A neutral atom has the same number of protons and electrons. By definition, an ion is an electrically charged. This number is the atomic number of the element. A helium atom that has lost or. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Helium (He Do Positive Ions Have The Same Number Of Protons And Electrons In nature, however, many atoms lose or gain electrons. Metal atoms lose electrons from their outer shell when they form ions: Recall that neutral atoms have the same number of protons and electrons. This number is the atomic number of the element. A helium atom that has lost or gained an electron is an ion. A neutral atom has the. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.slideserve.com
PPT Chapter 3 From Chemistry to Energy to Life PowerPoint Do Positive Ions Have The Same Number Of Protons And Electrons Recall that neutral atoms have the same number of protons and electrons. You can determine the number of electrons in an ion if you know. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. In many cases, elements that. This number is the atomic number of the element.. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.chegg.com
Solved Identify the ion with the given number of protons and Do Positive Ions Have The Same Number Of Protons And Electrons You can determine the number of electrons in an ion if you know. A neutral atom has the same number of protons and electrons. The atom is neutral as it has two positive protons and two negative electrons. They contain the same number of protons as electrons. This number is the atomic number of the element. Recall that neutral atoms. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.pinterest.com
Spectroscopy Electron configuration, Chemistry education, Protons Do Positive Ions Have The Same Number Of Protons And Electrons Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. A neutral atom has the same number of protons and electrons. By definition, an ion is an electrically charged. You can determine the number of electrons in an ion if you know. They contain the same number of protons. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Do Positive Ions Have The Same Number Of Protons And Electrons A helium atom that has lost or gained an electron is an ion. The atom is neutral as it has two positive protons and two negative electrons. By definition, an ion is an electrically charged. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. Metal atoms lose electrons. Do Positive Ions Have The Same Number Of Protons And Electrons.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Do Positive Ions Have The Same Number Of Protons And Electrons The atom is neutral as it has two positive protons and two negative electrons. By definition, an ion is an electrically charged. They contain the same number of protons as electrons. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. Metal atoms lose electrons from their outer shell. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.youtube.com
How to find the number of Protons, Neutrons and Electrons? Chemistry Do Positive Ions Have The Same Number Of Protons And Electrons Metal atoms lose electrons from their outer shell when they form ions: Recall that neutral atoms have the same number of protons and electrons. By definition, an ion is an electrically charged. The atom is neutral as it has two positive protons and two negative electrons. In many cases, elements that. A helium atom that has lost or gained an. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.wikihow.com
How to Find Electrons 7 Steps (with Pictures) wikiHow Do Positive Ions Have The Same Number Of Protons And Electrons The ions are positive, because they have more. A neutral atom has the same number of protons and electrons. The atom is neutral as it has two positive protons and two negative electrons. In nature, however, many atoms lose or gain electrons. A helium atom that has lost or gained an electron is an ion. They contain the same number. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.shalom-education.com
Isotopes and Ions GCSE Physics Revision Do Positive Ions Have The Same Number Of Protons And Electrons In nature, however, many atoms lose or gain electrons. They contain the same number of protons as electrons. The ions are positive, because they have more. A neutral atom has the same number of protons and electrons. You can determine the number of electrons in an ion if you know. This number is the atomic number of the element. Recall. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.wikihow.com
How to Find the Number of Protons, Neutrons, and Electrons Do Positive Ions Have The Same Number Of Protons And Electrons Metal atoms lose electrons from their outer shell when they form ions: A neutral atom has the same number of protons and electrons. The atom is neutral as it has two positive protons and two negative electrons. The ions are positive, because they have more. A helium atom that has lost or gained an electron is an ion. Recall that. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Do Positive Ions Have The Same Number Of Protons And Electrons The atom is neutral as it has two positive protons and two negative electrons. The atom is neutral as it has two positive protons and two negative electrons. By definition, an ion is an electrically charged. Metal atoms lose electrons from their outer shell when they form ions: A neutral atom has the same number of protons and electrons. They. Do Positive Ions Have The Same Number Of Protons And Electrons.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic Do Positive Ions Have The Same Number Of Protons And Electrons Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. Recall that neutral atoms have the same number of protons and electrons. A neutral atom has the same number of protons and electrons. In many cases, elements that. You can determine the number of electrons in an ion if. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.sliderbase.com
Isotopes. Do Positive Ions Have The Same Number Of Protons And Electrons Metal atoms lose electrons from their outer shell when they form ions: The ions are positive, because they have more. The atom is neutral as it has two positive protons and two negative electrons. A helium atom that has lost or gained an electron is an ion. By definition, an ion is an electrically charged. Recall that neutral atoms have. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.sliderbase.com
The Atom Presentation Chemistry Do Positive Ions Have The Same Number Of Protons And Electrons A helium atom that has lost or gained an electron is an ion. The atom is neutral as it has two positive protons and two negative electrons. A helium atom that has lost or gained an electron is an ion. In nature, however, many atoms lose or gain electrons. Recall that neutral atoms have the same number of protons and. Do Positive Ions Have The Same Number Of Protons And Electrons.
From sciencenotes.org
What Is an Ion? Chemistry Definition Do Positive Ions Have The Same Number Of Protons And Electrons They contain the same number of protons as electrons. The atom is neutral as it has two positive protons and two negative electrons. Metal atoms lose electrons from their outer shell when they form ions: The atom is neutral as it has two positive protons and two negative electrons. A neutral atom has the same number of protons and electrons.. Do Positive Ions Have The Same Number Of Protons And Electrons.
From room201csa.weebly.com
Atoms and Elements Científicos Matemáticos Do Positive Ions Have The Same Number Of Protons And Electrons They contain the same number of protons as electrons. A neutral atom has the same number of protons and electrons. The ions are positive, because they have more. A helium atom that has lost or gained an electron is an ion. The atom is neutral as it has two positive protons and two negative electrons. This number is the atomic. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.slideserve.com
PPT Atoms and Ions PowerPoint Presentation, free download ID2050882 Do Positive Ions Have The Same Number Of Protons And Electrons A helium atom that has lost or gained an electron is an ion. By definition, an ion is an electrically charged. This number is the atomic number of the element. The ions are positive, because they have more. Recall that neutral atoms have the same number of protons and electrons. The atom is neutral as it has two positive protons. Do Positive Ions Have The Same Number Of Protons And Electrons.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Do Positive Ions Have The Same Number Of Protons And Electrons Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. Metal atoms lose electrons from their outer shell when they form ions: This number is the atomic number of the element. By definition, an ion is an electrically charged. A helium atom that has lost or gained an electron. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent Do Positive Ions Have The Same Number Of Protons And Electrons In many cases, elements that. A helium atom that has lost or gained an electron is an ion. They contain the same number of protons as electrons. Metal atoms lose electrons from their outer shell when they form ions: In nature, however, many atoms lose or gain electrons. This number is the atomic number of the element. The atom is. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.slideserve.com
PPT ATOMIC STRUCTURE PowerPoint Presentation, free download ID357852 Do Positive Ions Have The Same Number Of Protons And Electrons This number is the atomic number of the element. The atom is neutral as it has two positive protons and two negative electrons. By definition, an ion is an electrically charged. They contain the same number of protons as electrons. You can determine the number of electrons in an ion if you know. The atom is neutral as it has. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.slideserve.com
PPT Ions Positively and Negatively charged atoms PowerPoint Do Positive Ions Have The Same Number Of Protons And Electrons You can determine the number of electrons in an ion if you know. By definition, an ion is an electrically charged. A neutral atom has the same number of protons and electrons. In nature, however, many atoms lose or gain electrons. In many cases, elements that. The atom is neutral as it has two positive protons and two negative electrons.. Do Positive Ions Have The Same Number Of Protons And Electrons.
From elchoroukhost.net
Periodic Table With Charges Of Ions Elcho Table Do Positive Ions Have The Same Number Of Protons And Electrons The ions are positive, because they have more. They contain the same number of protons as electrons. The atom is neutral as it has two positive protons and two negative electrons. You can determine the number of electrons in an ion if you know. In many cases, elements that. A neutral atom has the same number of protons and electrons.. Do Positive Ions Have The Same Number Of Protons And Electrons.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID4939543 Do Positive Ions Have The Same Number Of Protons And Electrons In nature, however, many atoms lose or gain electrons. Metal atoms lose electrons from their outer shell when they form ions: A helium atom that has lost or gained an electron is an ion. By definition, an ion is an electrically charged. The atom is neutral as it has two positive protons and two negative electrons. They contain the same. Do Positive Ions Have The Same Number Of Protons And Electrons.
From mungfali.com
Protons Neutrons And Electrons Diagram Do Positive Ions Have The Same Number Of Protons And Electrons Metal atoms lose electrons from their outer shell when they form ions: You can determine the number of electrons in an ion if you know. The atom is neutral as it has two positive protons and two negative electrons. In many cases, elements that. This number is the atomic number of the element. The ions are positive, because they have. Do Positive Ions Have The Same Number Of Protons And Electrons.
From schematicmandorlas.z14.web.core.windows.net
Understanding Protons Electrons And Neutrons Do Positive Ions Have The Same Number Of Protons And Electrons They contain the same number of protons as electrons. Metal atoms lose electrons from their outer shell when they form ions: The atom is neutral as it has two positive protons and two negative electrons. A helium atom that has lost or gained an electron is an ion. A helium atom that has lost or gained an electron is an. Do Positive Ions Have The Same Number Of Protons And Electrons.