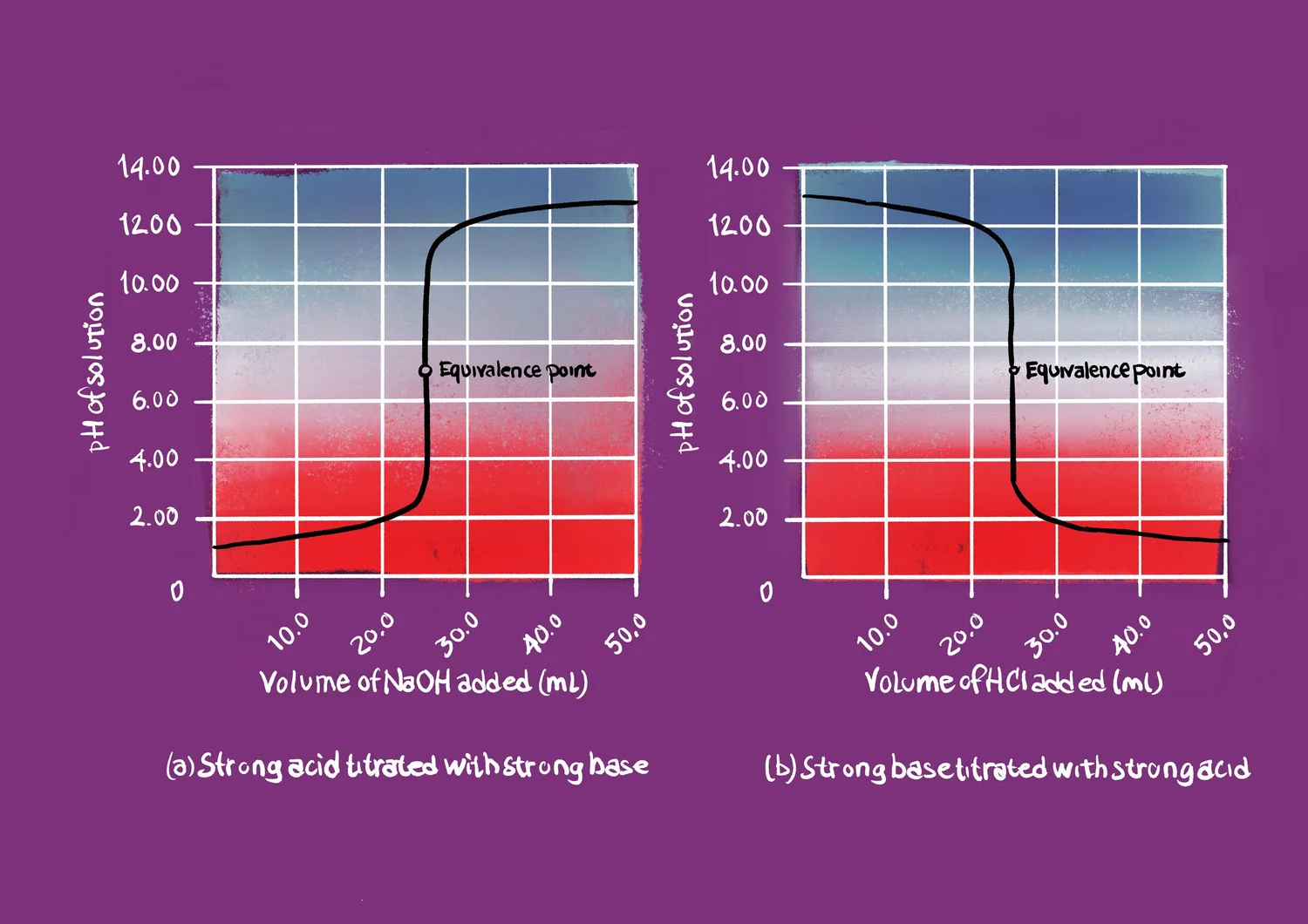
Acid-Base Titration Basic Steps . As seen in the chapter on the. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. there are two basic types of acid base titrations, indicator and potentiometric. Fill up a burette with acid. a titration curve is a plot of some solution property versus the amount of added titrant. compute sample ph at important stages of a titration. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. Understand the use of indicators. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Compute sample ph at important stages of a titration. The analyte (titrand) is the solution with an unknown molarity. the steps in the titration are as follows: the ph detection helps control food quality, prevent spoilage, determine storage methods, and monitor additive. In this article, we will discuss the. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a.
from ebinu.blog
The analyte (titrand) is the solution with an unknown molarity. it is known to determine the unknown concentration of an identified analyte. Compute sample ph at important stages of a titration. one point in the titration of a weak acid or a weak base is particularly important: In this article, we will discuss the. Through this process, an acid or base of known. the steps in the titration are as follows: As seen in the chapter on the. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. A burette is used to place the acid of unknown.
AcidBase Titration Lab — DataClassroom / Acid Base Titration
Acid-Base Titration Basic Steps The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. Understand the use of indicators. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. compute sample ph at important stages of a titration. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. the steps in the titration are as follows: Titration is a common laboratory technique for determining the concentration of a solute. Through this process, an acid or base of known. As seen in the chapter on the. the ph detection helps control food quality, prevent spoilage, determine storage methods, and monitor additive. Fill up a burette with acid. a titration curve is a plot of some solution property versus the amount of added titrant. it is known to determine the unknown concentration of an identified analyte. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. In this article, we will discuss the.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Acid-Base Titration Basic Steps Compute sample ph at important stages of a titration. Understand the use of indicators. it is known to determine the unknown concentration of an identified analyte. In this article, we will discuss the. The reagent (titrant) is the solution with a known molarity that will react with the analyte. As seen in the chapter on the. the process. Acid-Base Titration Basic Steps.
From ar.inspiredpencil.com
Acid Base Titration Acid-Base Titration Basic Steps one point in the titration of a weak acid or a weak base is particularly important: The reagent (titrant) is the solution with a known molarity that will react with the analyte. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. the ph detection helps control food quality,. Acid-Base Titration Basic Steps.
From mungfali.com
Acid Base Titration Calculation Acid-Base Titration Basic Steps The analyte (titrand) is the solution with an unknown molarity. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. the ph detection helps control food quality,. Acid-Base Titration Basic Steps.
From www.sliderbase.com
Titration Acid-Base Titration Basic Steps Understand the use of indicators. the steps in the titration are as follows: In this article, we will discuss the. the ph detection helps control food quality, prevent spoilage, determine storage methods, and monitor additive. a titration curve is a plot of some solution property versus the amount of added titrant. The reagent (titrant) is the solution. Acid-Base Titration Basic Steps.
From saylordotorg.github.io
AcidBase Titrations Acid-Base Titration Basic Steps it is known to determine the unknown concentration of an identified analyte. A burette is used to place the acid of unknown. Understand the use of indicators. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. Fill up a burette with acid. one point. Acid-Base Titration Basic Steps.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Acid-Base Titration Basic Steps Titration is a common laboratory technique for determining the concentration of a solute. compute sample ph at important stages of a titration. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. the process of obtaining quantitative information of a sample using a fast chemical. Acid-Base Titration Basic Steps.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Acid-Base Titration Basic Steps A burette is used to place the acid of unknown. The reagent (titrant) is the solution with a known molarity that will react with the analyte. one point in the titration of a weak acid or a weak base is particularly important: Titration is a common laboratory technique for determining the concentration of a solute. there are two. Acid-Base Titration Basic Steps.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Acid-Base Titration Basic Steps a titration curve is a plot of some solution property versus the amount of added titrant. In this article, we will discuss the. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. As seen in the chapter on the. Titration is a common laboratory technique. Acid-Base Titration Basic Steps.
From mungfali.com
Acid Base Titration Calculation Acid-Base Titration Basic Steps In this article, we will discuss the. the steps in the titration are as follows: Understand the use of indicators. compute sample ph at important stages of a titration. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. Through this process, an acid or base of known. . Acid-Base Titration Basic Steps.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Acid-Base Titration Basic Steps it is known to determine the unknown concentration of an identified analyte. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Through this process, an acid or base of known. In this article, we will discuss the. there are two basic types of acid base titrations, indicator and potentiometric. by. Acid-Base Titration Basic Steps.
From theedge.com.hk
Chemistry How To Titration The Edge Acid-Base Titration Basic Steps the steps in the titration are as follows: it is known to determine the unknown concentration of an identified analyte. Fill up a burette with acid. there are two basic types of acid base titrations, indicator and potentiometric. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a.. Acid-Base Titration Basic Steps.
From www.youtube.com
Introduction to Acid Base Titrations YouTube Acid-Base Titration Basic Steps Titration is a common laboratory technique for determining the concentration of a solute. As seen in the chapter on the. the steps in the titration are as follows: The analyte (titrand) is the solution with an unknown molarity. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. the. Acid-Base Titration Basic Steps.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Acid-Base Titration Basic Steps there are two basic types of acid base titrations, indicator and potentiometric. Understand the use of indicators. it is known to determine the unknown concentration of an identified analyte. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Fill up a burette with acid. one point. Acid-Base Titration Basic Steps.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Acid-Base Titration Basic Steps the ph detection helps control food quality, prevent spoilage, determine storage methods, and monitor additive. As seen in the chapter on the. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Through this process, an acid or base of known. the process of obtaining quantitative information of. Acid-Base Titration Basic Steps.
From ar.inspiredpencil.com
Titration Diagram Acid-Base Titration Basic Steps it is known to determine the unknown concentration of an identified analyte. Understand the use of indicators. there are two basic types of acid base titrations, indicator and potentiometric. A burette is used to place the acid of unknown. Compute sample ph at important stages of a titration. the ph detection helps control food quality, prevent spoilage,. Acid-Base Titration Basic Steps.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Acid-Base Titration Basic Steps Understand the use of indicators. A burette is used to place the acid of unknown. there are two basic types of acid base titrations, indicator and potentiometric. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Compute sample ph at important stages of a titration. Titration is a. Acid-Base Titration Basic Steps.
From saylordotorg.github.io
AcidBase Titrations Acid-Base Titration Basic Steps The reagent (titrant) is the solution with a known molarity that will react with the analyte. Understand the use of indicators. there are two basic types of acid base titrations, indicator and potentiometric. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. In this article, we will discuss. Acid-Base Titration Basic Steps.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Acid-Base Titration Basic Steps Fill up a burette with acid. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. A burette is used to place the acid of unknown. the ph detection helps control food quality, prevent spoilage, determine storage methods, and monitor additive. a titration curve is a plot of. Acid-Base Titration Basic Steps.
From facts.net
8 Captivating Facts About AcidBase Titration Acid-Base Titration Basic Steps As seen in the chapter on the. it is known to determine the unknown concentration of an identified analyte. there are two basic types of acid base titrations, indicator and potentiometric. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. In this article, we. Acid-Base Titration Basic Steps.
From mungfali.com
Acid Base Titration Procedure Acid-Base Titration Basic Steps Through this process, an acid or base of known. Titration is a common laboratory technique for determining the concentration of a solute. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. It is based on the neutralization reaction, where an acid and a base react to. Acid-Base Titration Basic Steps.
From ebinu.blog
AcidBase Titration Lab — DataClassroom / Acid Base Titration Acid-Base Titration Basic Steps Fill up a burette with acid. compute sample ph at important stages of a titration. it is known to determine the unknown concentration of an identified analyte. the steps in the titration are as follows: Understand the use of indicators. The analyte (titrand) is the solution with an unknown molarity. It is based on the neutralization reaction,. Acid-Base Titration Basic Steps.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Acid-Base Titration Basic Steps In this article, we will discuss the. one point in the titration of a weak acid or a weak base is particularly important: Compute sample ph at important stages of a titration. compute sample ph at important stages of a titration. the steps in the titration are as follows: it is known to determine the unknown. Acid-Base Titration Basic Steps.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Acid-Base Titration Basic Steps Through this process, an acid or base of known. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. A burette is used to place the acid of unknown. As seen in the chapter on the. the ph detection helps control food quality, prevent spoilage, determine storage methods, and. Acid-Base Titration Basic Steps.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Acid-Base Titration Basic Steps In this article, we will discuss the. The analyte (titrand) is the solution with an unknown molarity. As seen in the chapter on the. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. one point in the titration of a weak acid or a weak base is particularly important:. Acid-Base Titration Basic Steps.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid-Base Titration Basic Steps In this article, we will discuss the. compute sample ph at important stages of a titration. Titration is a common laboratory technique for determining the concentration of a solute. Compute sample ph at important stages of a titration. As seen in the chapter on the. Understand the use of indicators. the ph detection helps control food quality, prevent. Acid-Base Titration Basic Steps.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Acid-Base Titration Basic Steps it is known to determine the unknown concentration of an identified analyte. there are two basic types of acid base titrations, indicator and potentiometric. Fill up a burette with acid. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. It is based on the neutralization reaction, where an. Acid-Base Titration Basic Steps.
From mungfali.com
Acid Base Titration Method Acid-Base Titration Basic Steps Titration is a common laboratory technique for determining the concentration of a solute. one point in the titration of a weak acid or a weak base is particularly important: a titration curve is a plot of some solution property versus the amount of added titrant. The reagent (titrant) is the solution with a known molarity that will react. Acid-Base Titration Basic Steps.
From mungfali.com
Acid Base Titration Procedure Acid-Base Titration Basic Steps As seen in the chapter on the. The reagent (titrant) is the solution with a known molarity that will react with the analyte. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Understand the use of indicators. Compute sample ph at important stages of a titration. In this article,. Acid-Base Titration Basic Steps.
From www.biorender.com
AcidBase Titration BioRender Science Templates Acid-Base Titration Basic Steps it is known to determine the unknown concentration of an identified analyte. the steps in the titration are as follows: Understand the use of indicators. Titration is a common laboratory technique for determining the concentration of a solute. In this article, we will discuss the. Fill up a burette with acid. Through this process, an acid or base. Acid-Base Titration Basic Steps.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Acid-Base Titration Basic Steps the steps in the titration are as follows: In this article, we will discuss the. Fill up a burette with acid. the process of obtaining quantitative information of a sample using a fast chemical reaction by reacting with a. As seen in the chapter on the. It is based on the neutralization reaction, where an acid and a. Acid-Base Titration Basic Steps.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle Acid-Base Titration Basic Steps the ph detection helps control food quality, prevent spoilage, determine storage methods, and monitor additive. one point in the titration of a weak acid or a weak base is particularly important: The reagent (titrant) is the solution with a known molarity that will react with the analyte. by determining the volume of strong based needed to reach. Acid-Base Titration Basic Steps.
From saylordotorg.github.io
AcidBase Titrations Acid-Base Titration Basic Steps it is known to determine the unknown concentration of an identified analyte. the ph detection helps control food quality, prevent spoilage, determine storage methods, and monitor additive. Understand the use of indicators. The reagent (titrant) is the solution with a known molarity that will react with the analyte. one point in the titration of a weak acid. Acid-Base Titration Basic Steps.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Acid-Base Titration Basic Steps As seen in the chapter on the. Titration is a common laboratory technique for determining the concentration of a solute. it is known to determine the unknown concentration of an identified analyte. there are two basic types of acid base titrations, indicator and potentiometric. Compute sample ph at important stages of a titration. In this article, we will. Acid-Base Titration Basic Steps.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Acid-Base Titration Basic Steps the steps in the titration are as follows: In this article, we will discuss the. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The. Acid-Base Titration Basic Steps.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Acid-Base Titration Basic Steps the ph detection helps control food quality, prevent spoilage, determine storage methods, and monitor additive. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. compute sample ph at important stages of a titration. the process of obtaining quantitative information of a sample using. Acid-Base Titration Basic Steps.