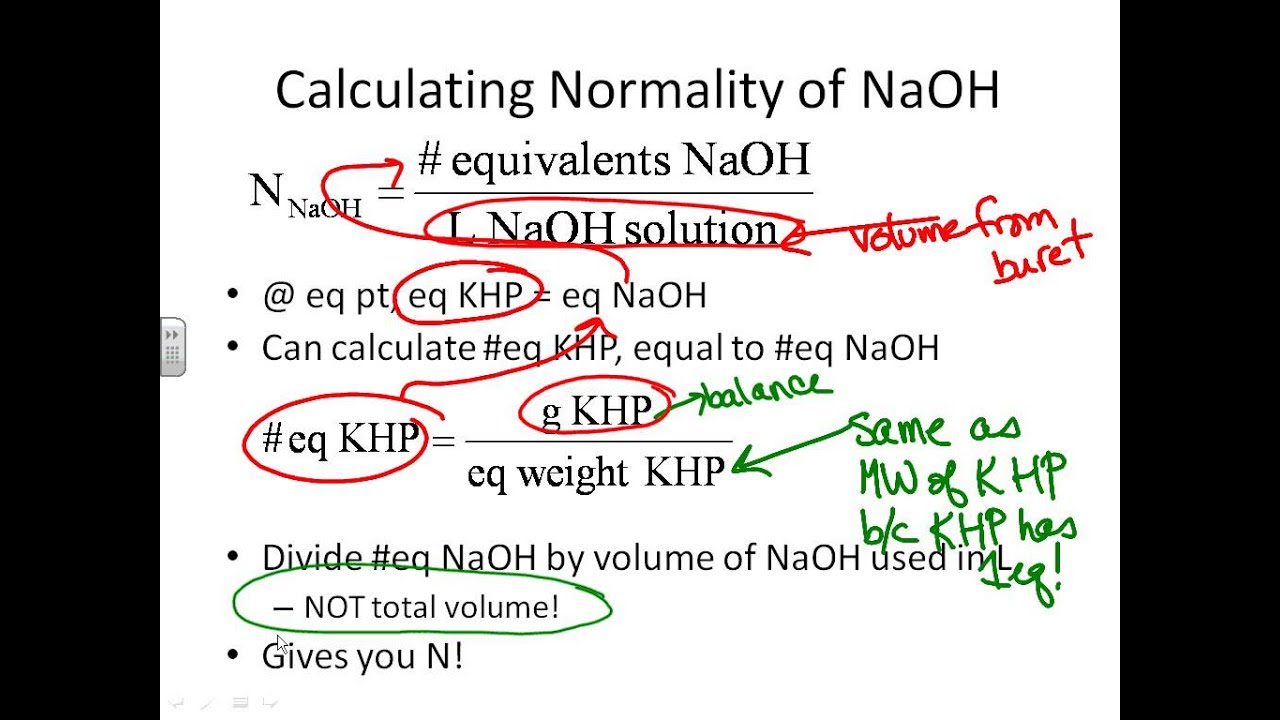
Titration Standardization Of Naoh . Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. A typical titration setup is illustrated in the figure below. Apply basic statistics to a data set to. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. In the first standardization the molarity of a sodium. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00.
from www.youtube.com
Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. A typical titration setup is illustrated in the figure below. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. Apply basic statistics to a data set to. In the first standardization the molarity of a sodium. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures.
Prelab for NaOH Standardization lab YouTube
Titration Standardization Of Naoh To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. A typical titration setup is illustrated in the figure below. In the first standardization the molarity of a sodium. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. Apply basic statistics to a data set to. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Standardization Of Naoh In the first standardization the molarity of a sodium. Apply basic statistics to a data set to. A typical titration setup is illustrated in the figure below. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating. Titration Standardization Of Naoh.
From www.numerade.com
SOLVED Standardization of NaOH Name Part I Titrant Preparation Titration Standardization Of Naoh Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. Apply basic statistics to a data set to. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant. Titration Standardization Of Naoh.
From www.chegg.com
Solved TITRATION ⋅ STANDARDIZATION OF SODIUM HYDROXIDE Titration Standardization Of Naoh To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. A typical titration setup is illustrated in the. Titration Standardization Of Naoh.
From www.chegg.com
Solved Titration Standardization of sodium hydroxide (NaOH) Titration Standardization Of Naoh Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. In the first standardization the molarity of a sodium. Use this prepared primary standard to. Titration Standardization Of Naoh.
From www.chegg.com
Solved TITRATION. STANDARDIZATION OF SODIUM HYDROXIDE Titration Standardization Of Naoh To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. Apply basic statistics to a data set to. A typical titration setup is illustrated in the figure below. In the first. Titration Standardization Of Naoh.
From www.scribd.com
Standardization of a Base Titration Sodium Hydroxide Titration Standardization Of Naoh Apply basic statistics to a data set to. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. In the first standardization the molarity of a sodium. In order to determine the exact concentration of a sodium hydroxide. Titration Standardization Of Naoh.
From www.scribd.com
Standardization of Naoh PDF Titration Chemistry Titration Standardization Of Naoh To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. Use. Titration Standardization Of Naoh.
From www.chegg.com
Solved TITRATION • STANDARDIZATION OF SODIUM HYDROXIDE Titration Standardization Of Naoh In the first standardization the molarity of a sodium. Apply basic statistics to a data set to. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that. Titration Standardization Of Naoh.
From www.chegg.com
TitrationStandardization of Sodium Hydroxide Titration Standardization Of Naoh Apply basic statistics to a data set to. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. In the first standardization the molarity of a sodium. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid. Titration Standardization Of Naoh.
From www.numerade.com
SOLVED Experiment 1 AcidBase Titration Standardization of NaOH Titration Standardization Of Naoh In the first standardization the molarity of a sodium. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. Apply basic statistics to a data set to. Standardizing a sodium hydroxide (naoh) solution in a titration, it is. Titration Standardization Of Naoh.
From chemistrypubs.com
Standardization of HCl with standard NaOH solution Titration Standardization Of Naoh Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. To standardize a. Titration Standardization Of Naoh.
From www.youtube.com
Chem 60 Experiment 10 Part 1 Standardization of NaOH Solution YouTube Titration Standardization Of Naoh In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant. Titration Standardization Of Naoh.
From studylib.net
CHM 115 Lab 3 Titration Standardize NaOH/Determine impure KHP Titration Standardization Of Naoh Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. In the first standardization the molarity of a sodium. Use this prepared primary standard to accurately standardize a naoh solution to. Titration Standardization Of Naoh.
From www.chegg.com
Solved TITRATION STANDARDIZATION OF SODIUM HYDROXIDE SUBMIT Titration Standardization Of Naoh Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. In order to. Titration Standardization Of Naoh.
From www.numerade.com
SOLVED Part III Titration Standardization of NaOH solution (finding Titration Standardization Of Naoh Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. Apply basic statistics to a data set to. In the first standardization the molarity of a sodium. A typical titration setup is illustrated in the. Titration Standardization Of Naoh.
From www.youtube.com
Animation Titration Preparation and Standardization of 1M Sodium Titration Standardization Of Naoh In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. In the first standardization the molarity of a sodium. A typical titration setup is illustrated in the figure below. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. Titration Standardization Of Naoh.
From www.numerade.com
SOLVED What is the concentration of NaOH? TITRATION STANDARDIZATION OF Titration Standardization Of Naoh In the first standardization the molarity of a sodium. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. Apply basic statistics to a data set to. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Standardizing. Titration Standardization Of Naoh.
From www.vrogue.co
Standardization Of Naoh With A Khp Solution Acid Base vrogue.co Titration Standardization Of Naoh Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. In the first standardization the molarity of a sodium. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)]. Titration Standardization Of Naoh.
From www.slideserve.com
PPT Titrations PowerPoint Presentation ID2145156 Titration Standardization Of Naoh To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. In the first standardization the molarity of a sodium. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. A typical titration setup is illustrated in the figure. Titration Standardization Of Naoh.
From www.visionlearning.com
Acids and Bases I Math in Science Visionlearning Titration Standardization Of Naoh To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. Use the virtual. Titration Standardization Of Naoh.
From www.slideserve.com
PPT Titration Expt. Overview PowerPoint Presentation, free download Titration Standardization Of Naoh To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. A typical titration setup is illustrated in the. Titration Standardization Of Naoh.
From www.chegg.com
Solved TITRATION STANDARDIZATION OF SODIUM HYDROXIDE Titration Standardization Of Naoh In the first standardization the molarity of a sodium. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with. Titration Standardization Of Naoh.
From www.scribd.com
06 and 07 Standardization of NaOH and Acid Base Titration (1 Titration Standardization Of Naoh Apply basic statistics to a data set to. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. A typical titration setup is illustrated in the figure below. In the first standardization the molarity of a sodium. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to. Titration Standardization Of Naoh.
From www.numerade.com
TITRATION STANDARDIZATION OF SODIUM HYDROXIDE INTRODUCTION LABORATORY Titration Standardization Of Naoh Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures.. Titration Standardization Of Naoh.
From www.numerade.com
SOLVED Part II Titration Standardization of NaOH Solution (Finding Titration Standardization Of Naoh In the first standardization the molarity of a sodium. Apply basic statistics to a data set to. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. A typical titration. Titration Standardization Of Naoh.
From www.numerade.com
SOLVED Question 3 Please Experiment 5 AcidBase Titration Titration Standardization Of Naoh Apply basic statistics to a data set to. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. A typical titration setup is illustrated in the figure below. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. To standardize. Titration Standardization Of Naoh.
From www.youtube.com
Standardization of NaOH by titration using KHP YouTube Titration Standardization Of Naoh A typical titration setup is illustrated in the figure below. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. In the first standardization the molarity of a sodium. Apply basic statistics to a data set to. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to. Titration Standardization Of Naoh.
From www.youtube.com
Prelab for NaOH Standardization lab YouTube Titration Standardization Of Naoh In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. A typical titration setup is illustrated in the figure below. Apply basic statistics to a data set to. To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using.. Titration Standardization Of Naoh.
From www.chegg.com
Solved TITRATION. STANDARDIZATION OF SODIUM HYDROXIDE Titration Standardization Of Naoh Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. Apply basic statistics to a data set to. Use the virtual laboratory to. Titration Standardization Of Naoh.
From www.studypool.com
SOLUTION Laboratory Report Standardization of NaOH with KHP and Titration Standardization Of Naoh To standardize a sodium hydroxide (naoh) solution against a primary standard acid [potassium hydrogen phthalate (khp)] using. Apply basic statistics to a data set to. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant. Titration Standardization Of Naoh.
From www.chegg.com
Solved TITRATION. STANDARDIZATION OF SODIUM HYDROXIDE SUBMIT Titration Standardization Of Naoh Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. Apply basic statistics to a data set to. A typical titration setup is illustrated in the figure below. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a. Titration Standardization Of Naoh.
From mavink.com
Titration Reaction Titration Standardization Of Naoh Apply basic statistics to a data set to. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. In the first standardization the. Titration Standardization Of Naoh.
From www.vrogue.co
Standardization Of Naoh Solution Preparing Standard S vrogue.co Titration Standardization Of Naoh Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the exact concentration of the titrant (the solution. In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. Use this prepared primary standard to accurately standardize a naoh solution to four. Titration Standardization Of Naoh.
From www.youtube.com
Mass of KHP to Standardize a NaOH Solution YouTube Titration Standardization Of Naoh In order to determine the exact concentration of a sodium hydroxide solution you must standardize it by titrating with a solid acid that is not. A typical titration setup is illustrated in the figure below. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. Standardizing a sodium hydroxide (naoh). Titration Standardization Of Naoh.
From www.vrogue.co
Standardization Of Naoh Acid Base Titration Objective vrogue.co Titration Standardization Of Naoh A typical titration setup is illustrated in the figure below. Use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via titration with 25.00. Use this prepared primary standard to accurately standardize a naoh solution to four significant figures. Standardizing a sodium hydroxide (naoh) solution in a titration, it is critical to know the. Titration Standardization Of Naoh.