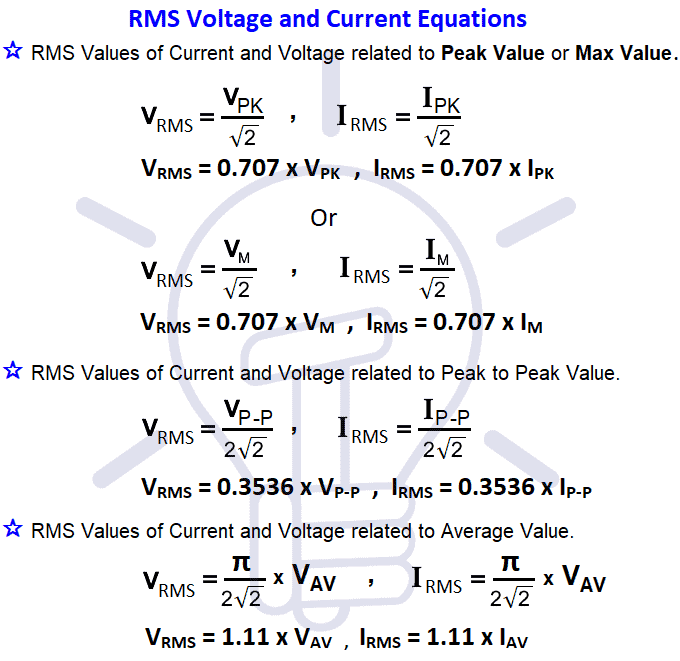What Is The Value Of K When E 0 V . The k value is very large, indicating. Also, when e°= 0 v, k is always 1. The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. Only values of \(δg^o =. The k sp is determined directly from the electrochemical data. Express your answer using one significant figure. At equilibrium q = k. Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. Because products are in the. The logarithm (with any base) of 0 is always 1. Substituting in k for q, and the values for r, t, and f, we get: What is the value of k when e degree = 0v? Therefore, when k < 1, e° is always negative. Using the following reduction potentials, calculate the.
from www.electricaltechnology.org
The k sp is determined directly from the electrochemical data. What is the value of k when e degree = 0v? Substituting in k for q, and the values for r, t, and f, we get: Using the following reduction potentials, calculate the. Express your answer using one significant figure. At equilibrium q = k. Therefore, when k < 1, e° is always negative. The k value is very large, indicating. Because products are in the. The logarithm (with any base) of 0 is always 1.
RMS Value, Average Value, Peak Value, Peak Factor, Form Factor in AC
What Is The Value Of K When E 0 V Substituting in k for q, and the values for r, t, and f, we get: Using the following reduction potentials, calculate the. Also, when e°= 0 v, k is always 1. The k sp is determined directly from the electrochemical data. What is the value of k when e degree = 0v? Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. Because products are in the. The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. The k value is very large, indicating. Substituting in k for q, and the values for r, t, and f, we get: Only values of \(δg^o =. At equilibrium q = k. The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. The logarithm (with any base) of 0 is always 1. Express your answer using one significant figure. Therefore, when k < 1, e° is always negative.
From prntbl.concejomunicipaldechinu.gov.co
Find The Exact Value Of Each Trigonometric Function Worksheet prntbl What Is The Value Of K When E 0 V The logarithm (with any base) of 0 is always 1. The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. Using the following reduction potentials, calculate the. What is the value of k when e degree = 0v? Express your answer using one significant figure. Because products are in the. The. What Is The Value Of K When E 0 V.
From haipernews.com
How To Calculate Equilibrium Constant K Haiper What Is The Value Of K When E 0 V What is the value of k when e degree = 0v? Therefore, when k < 1, e° is always negative. The logarithm (with any base) of 0 is always 1. Using the following reduction potentials, calculate the. The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. Find the value of the equilibrium. What Is The Value Of K When E 0 V.
From www.vedantu.com
Decimals Definition, Facts and Examples What Is The Value Of K When E 0 V Using the following reduction potentials, calculate the. Only values of \(δg^o =. The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. Also, when e°= 0 v, k is always 1. Express your answer using one significant figure. Because products are in the. The logarithm (with any base) of 0 is. What Is The Value Of K When E 0 V.
From www.chegg.com
Solved Testing the Difference Between Two Means In Exercises What Is The Value Of K When E 0 V Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. Substituting in k for q, and the values for r, t, and f, we get: Also, when e°= 0 v, k is always 1. The k sp is determined directly from the electrochemical data. Only values of \(δg^o =. The k. What Is The Value Of K When E 0 V.
From www.youtube.com
Find value of constant k for the vectors to be perpendicular MCV4U TEST What Is The Value Of K When E 0 V Because products are in the. At equilibrium q = k. The k value is very large, indicating. The k sp is determined directly from the electrochemical data. Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. Only values of \(δg^o =. The logarithm (with any base) of 0 is always. What Is The Value Of K When E 0 V.
From www.statology.org
How to Find the Indicated Area Under the Standard Normal Curve What Is The Value Of K When E 0 V Express your answer using one significant figure. Using the following reduction potentials, calculate the. The k value is very large, indicating. The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. Also, when e°= 0 v, k is always 1. At equilibrium q = k. Therefore, when k < 1, e°. What Is The Value Of K When E 0 V.
From userdiagramdreich.z21.web.core.windows.net
Absolute Value Function Example What Is The Value Of K When E 0 V What is the value of k when e degree = 0v? The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. Also, when e°= 0 v, k is always 1. Express your answer using one significant figure. Using the following reduction potentials, calculate the. Only values of \(δg^o =. Substituting in. What Is The Value Of K When E 0 V.
From www.youtube.com
Value, Unit & Dimension of Epsilon naught & K (in Coulomb's Law What Is The Value Of K When E 0 V The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. Therefore, when k < 1, e° is always negative. Using the following reduction potentials, calculate the. The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. The logarithm (with any base) of 0 is. What Is The Value Of K When E 0 V.
From www.coursehero.com
[Solved] The equilibrium constant, Kc, for the reaction N2O4(g)⇌2NO2(g What Is The Value Of K When E 0 V Substituting in k for q, and the values for r, t, and f, we get: The k value is very large, indicating. Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude.. What Is The Value Of K When E 0 V.
From iq.opengenus.org
Confidence intervals What Is The Value Of K When E 0 V The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. At equilibrium q = k. Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. What is the value of k when e degree = 0v? Using the following reduction potentials, calculate the. Only. What Is The Value Of K When E 0 V.
From fyowivfoo.blob.core.windows.net
What Is The K Value In Statistics at Roberto Fulton blog What Is The Value Of K When E 0 V The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. Because products are in the. Using the following reduction potentials, calculate the. The k value is very large, indicating. Express your answer using one. What Is The Value Of K When E 0 V.
From solvedlib.com
What is the value of k such that u = [12] is orthogon… SolvedLib What Is The Value Of K When E 0 V The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. Using the following reduction potentials, calculate the. Also, when e°= 0 v, k is always 1. Express your answer using one significant. What Is The Value Of K When E 0 V.
From www.youtube.com
Initial Value Theorem YouTube What Is The Value Of K When E 0 V The k sp is determined directly from the electrochemical data. At equilibrium q = k. What is the value of k when e degree = 0v? The logarithm (with any base) of 0 is always 1. Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. Also, when e°= 0 v,. What Is The Value Of K When E 0 V.
From gionuoovg.blob.core.windows.net
What Is K Value Statistics at Toby Kunkle blog What Is The Value Of K When E 0 V Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. Substituting in k for q, and the values for r, t, and f, we get: Therefore, when k < 1, e° is always negative. The logarithm (with any base) of 0 is always 1. Also, when e°= 0 v, k is. What Is The Value Of K When E 0 V.
From www.hotzxgirl.com
Trigonometric Table Of All Angle Trigonometric Identities Hot Sex Picture What Is The Value Of K When E 0 V Using the following reduction potentials, calculate the. Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. Therefore, when k < 1, e° is always negative. Also, when e°= 0 v, k is always 1. Only values of \(δg^o =. What is the value of k when e degree = 0v?. What Is The Value Of K When E 0 V.
From medium.com
Poker Test (Sequence Randomness). Simulation and Modeling — Testing What Is The Value Of K When E 0 V Therefore, when k < 1, e° is always negative. The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. Because products are in the. Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. Express your answer using one significant figure. The k sp. What Is The Value Of K When E 0 V.
From ja.fmuser.net
コンデンサー電子FMUSERFM / TV放送ワンストップサプライヤーの価値を読み取るための非常に簡単な方法 What Is The Value Of K When E 0 V The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. The logarithm (with any base) of 0 is always 1. What is the value of k when e degree = 0v? Therefore, when k < 1, e° is always negative. Express your answer using one significant figure. The k value is very large,. What Is The Value Of K When E 0 V.
From exowevfjx.blob.core.windows.net
Ductwork K Values at Forrest Tomlinson blog What Is The Value Of K When E 0 V Therefore, when k < 1, e° is always negative. Also, when e°= 0 v, k is always 1. Express your answer using one significant figure. At equilibrium q = k. The logarithm (with any base) of 0 is always 1. Substituting in k for q, and the values for r, t, and f, we get: Only values of \(δg^o =.. What Is The Value Of K When E 0 V.
From elchoroukhost.net
Table Of Exact Trigonometric Values Elcho Table What Is The Value Of K When E 0 V Therefore, when k < 1, e° is always negative. The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. At equilibrium q = k. Only values of \(δg^o =. The k sp is determined directly from the electrochemical data. Because products are in the. Find the value of the equilibrium constant at 25. What Is The Value Of K When E 0 V.
From www.chegg.com
Solved 1. The Npoint Discrete Fourier Transform (DFT) can What Is The Value Of K When E 0 V Using the following reduction potentials, calculate the. Express your answer using one significant figure. Substituting in k for q, and the values for r, t, and f, we get: The k value is very large, indicating. The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. The logarithm (with any base). What Is The Value Of K When E 0 V.
From www.dummies.com
Finding Appropriate z* Values for Given Confidence Levels dummies What Is The Value Of K When E 0 V Express your answer using one significant figure. Using the following reduction potentials, calculate the. At equilibrium q = k. Only values of \(δg^o =. The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. Also, when e°= 0 v, k is always 1. Because products are in the. What is the value of. What Is The Value Of K When E 0 V.
From brokeasshome.com
trigonometry table pdf What Is The Value Of K When E 0 V The k sp is determined directly from the electrochemical data. The logarithm (with any base) of 0 is always 1. What is the value of k when e degree = 0v? The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. Find the value of the equilibrium constant at 25 o c for. What Is The Value Of K When E 0 V.
From www.youtube.com
Find the value of X Solve the given Equation and Inequality YouTube What Is The Value Of K When E 0 V The k value is very large, indicating. The logarithm (with any base) of 0 is always 1. The k sp is determined directly from the electrochemical data. Using the following reduction potentials, calculate the. The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. The values of \ (k\) shown in table \. What Is The Value Of K When E 0 V.
From sites.google.com
Day 70 Chi Charts David Bird Science What Is The Value Of K When E 0 V The logarithm (with any base) of 0 is always 1. The k value is very large, indicating. Because products are in the. Using the following reduction potentials, calculate the. The k sp is determined directly from the electrochemical data. Only values of \(δg^o =. Therefore, when k < 1, e° is always negative. Find the value of the equilibrium constant. What Is The Value Of K When E 0 V.
From www.youtube.com
Heat Transfer L6 p5 RValue and Thermal Resistance YouTube What Is The Value Of K When E 0 V Also, when e°= 0 v, k is always 1. Express your answer using one significant figure. What is the value of k when e degree = 0v? Therefore, when k < 1, e° is always negative. The k sp is determined directly from the electrochemical data. Because products are in the. Using the following reduction potentials, calculate the. Find the. What Is The Value Of K When E 0 V.
From www.tessshebaylo.com
How To Find X Value In Quadratic Equation Tessshebaylo What Is The Value Of K When E 0 V Because products are in the. The k sp is determined directly from the electrochemical data. The logarithm (with any base) of 0 is always 1. The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. The k value is very large, indicating. At equilibrium q = k. What is the value. What Is The Value Of K When E 0 V.
From mungfali.com
K Means Graph What Is The Value Of K When E 0 V Only values of \(δg^o =. The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. Also, when e°= 0 v, k is always 1. At equilibrium q = k. Using the following reduction potentials, calculate the. What is the value of k when e degree = 0v? Because products are in. What Is The Value Of K When E 0 V.
From www.chegg.com
Solved . I the Determine the values of k such that system What Is The Value Of K When E 0 V The logarithm (with any base) of 0 is always 1. Only values of \(δg^o =. Substituting in k for q, and the values for r, t, and f, we get: Express your answer using one significant figure. What is the value of k when e degree = 0v? Therefore, when k < 1, e° is always negative. The values of. What Is The Value Of K When E 0 V.
From www.electricaltechnology.org
RMS Value, Average Value, Peak Value, Peak Factor, Form Factor in AC What Is The Value Of K When E 0 V Using the following reduction potentials, calculate the. What is the value of k when e degree = 0v? Also, when e°= 0 v, k is always 1. The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. The logarithm (with any base) of 0 is always 1. Find the value of the equilibrium. What Is The Value Of K When E 0 V.
From www.youtube.com
For What value of k, the following pair equations has no solution 2x+3y What Is The Value Of K When E 0 V Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. The logarithm (with any base) of 0 is always 1. The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. The k sp is determined directly from the electrochemical data. Therefore, when. What Is The Value Of K When E 0 V.
From byjus.com
Epsilon Naught Value Definition, Units, Formula At BYJU’S What Is The Value Of K When E 0 V The k value is very large, indicating. The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. What is the value of k when e degree = 0v? Using the following reduction potentials, calculate the. Also, when e°= 0 v, k is always 1. The k sp is determined directly from. What Is The Value Of K When E 0 V.
From www.geeksforgeeks.org
Tan 30 Degrees (Tan 30°) Find the Value of Tan 30 Degrees What Is The Value Of K When E 0 V Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. The reaction is spontaneous, as indicated by a negative free energy change and a positive cell potential. The values of \ (k\) shown in table \ (\pageindex {2}\), for example, vary by 60 orders of magnitude. The logarithm (with any base). What Is The Value Of K When E 0 V.
From brainly.in
Find the value of k for which the quadratic equation 2x^2kx+k=0 has What Is The Value Of K When E 0 V Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. The logarithm (with any base) of 0 is always 1. What is the value of k when e degree = 0v? Using the following reduction potentials, calculate the. Also, when e°= 0 v, k is always 1. Only values of \(δg^o. What Is The Value Of K When E 0 V.
From spmaddmaths.blog.onlinetuition.com.my
3.3.1 Example 1 Finding the maximum/minimum and axis of symmetry of a What Is The Value Of K When E 0 V Express your answer using one significant figure. Because products are in the. The logarithm (with any base) of 0 is always 1. Using the following reduction potentials, calculate the. Find the value of the equilibrium constant at 25 o c for the cell reaction for the following electrochemical. The k sp is determined directly from the electrochemical data. The values. What Is The Value Of K When E 0 V.
From articles.outlier.org
How To Find Critical Value In Statistics Outlier What Is The Value Of K When E 0 V The logarithm (with any base) of 0 is always 1. Express your answer using one significant figure. The k sp is determined directly from the electrochemical data. Also, when e°= 0 v, k is always 1. At equilibrium q = k. Therefore, when k < 1, e° is always negative. Substituting in k for q, and the values for r,. What Is The Value Of K When E 0 V.