Device Deficiency Reporting . which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. mandatory medical device reporting: this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for.
from www.templateroller.com
if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. mandatory medical device reporting: The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory.
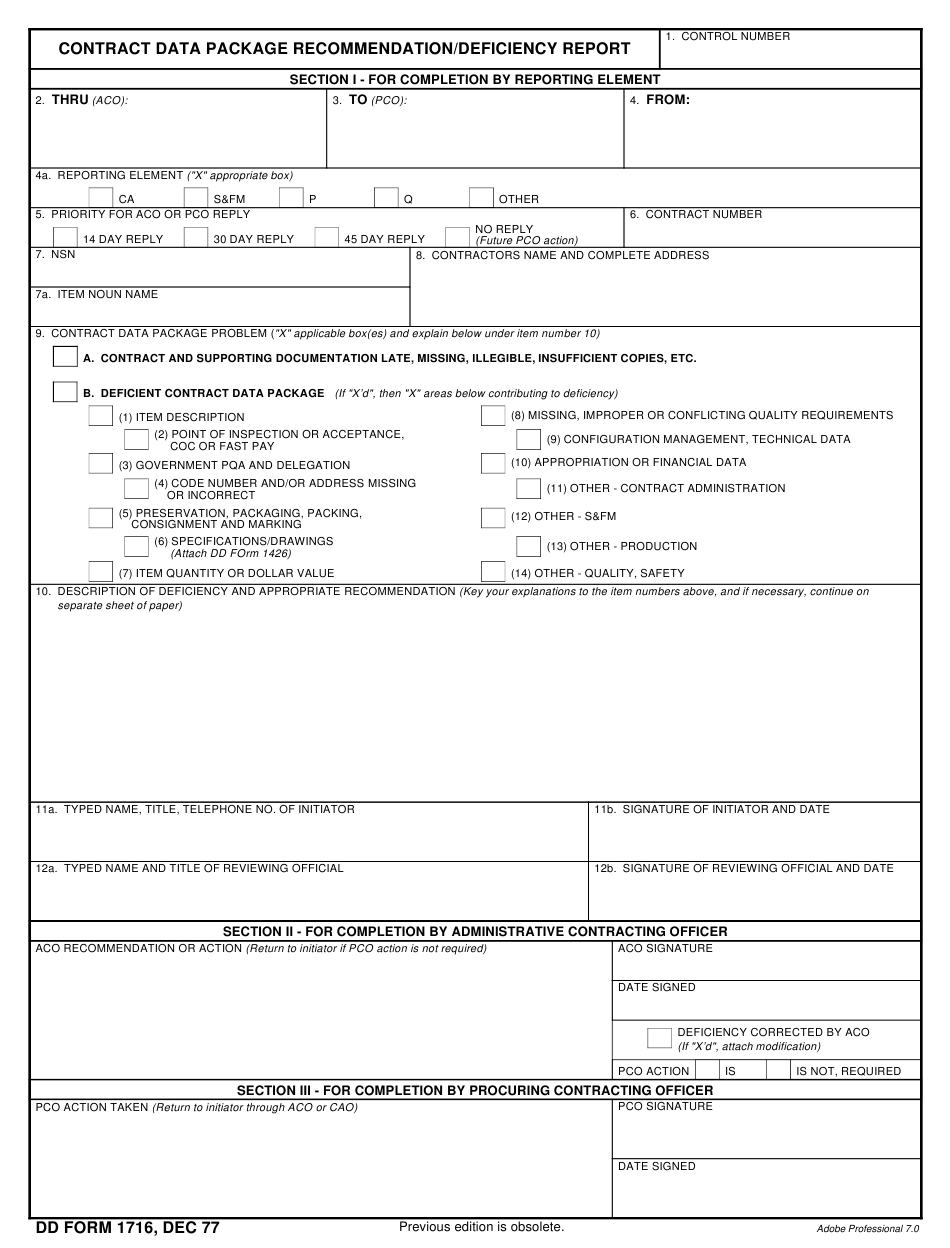
DD Form 1716 Download Fillable PDF or Fill Online Contract Data Package
Device Deficiency Reporting which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). mandatory medical device reporting: which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or.
From www.supremeschoolsupply.com
Deficiency Report, Triplicate (DR1) Supreme School Supply Device Deficiency Reporting mandatory medical device reporting: if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements. Device Deficiency Reporting.
From support.inspectpoint.com
How to Use Deficiency Report Notes Inspect Point Help Desk Device Deficiency Reporting which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. mandatory medical device reporting: the medical device reporting. Device Deficiency Reporting.
From www.researchgate.net
Medical device Adverse Event reporting form. Download Scientific Diagram Device Deficiency Reporting the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. mandatory medical device reporting: the medical device reporting (mdr) regulation. Device Deficiency Reporting.
From www.pdffiller.com
Device Deficiency Nonsubject related. Clinical Investigations with Device Deficiency Reporting the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. mandatory medical device reporting:. Device Deficiency Reporting.
From www.signnow.com
Deficiency Report Template 20102024 Form Fill Out and Sign Printable Device Deficiency Reporting this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. the medical device. Device Deficiency Reporting.
From www.researchgate.net
Medical device Adverse Event reporting form. Download Scientific Diagram Device Deficiency Reporting the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. if you are a device user facility, you must. Device Deficiency Reporting.
From druganddevicedigest.com
FDA Guidances Medical Device Reporting, Patient Connection Program Device Deficiency Reporting if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. the guidance document safety reporting in clinical investigations. Device Deficiency Reporting.
From hxeghawia.blob.core.windows.net
Medical Device Deficiency at Alan Insley blog Device Deficiency Reporting this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. which reporting forms are to be used. Device Deficiency Reporting.
From support.walshqa.com
Deficiency Report Walsh Integrated Knowledge Base Device Deficiency Reporting the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. mandatory medical device reporting:. Device Deficiency Reporting.
From navyaviation.tpub.com
Technical Publication Deficiency Report (TPDR), OPNAV 4790/66 Device Deficiency Reporting mandatory medical device reporting: the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. this document addresses good clinical practice for the design, conduct, recording and reporting of clinical. Device Deficiency Reporting.
From www.thepremedscene.com
Medical Device Deficiency Device Deficiency Reporting which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. mandatory medical device reporting: the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. if you are a device user facility, you must report deaths and serious injuries that a. Device Deficiency Reporting.
From present5.com
An Introduction to the Joint Deficiency Reporting System Device Deficiency Reporting this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. mandatory medical device reporting:. Device Deficiency Reporting.
From support.inspectpoint.com
How to Use Deficiency Report Notes Inspect Point Help Desk Device Deficiency Reporting the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd). Device Deficiency Reporting.
From support.walshqa.com
Deficiency Report Walsh Integrated Knowledge Base Device Deficiency Reporting the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. The medical device reporting. Device Deficiency Reporting.
From www.greenlight.guru
Ultimate Guide to ISO 141552020 for Medical Devices Device Deficiency Reporting the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for.. Device Deficiency Reporting.
From www.researchgate.net
Characteristics of the patients with isolated GH deficiency. Download Device Deficiency Reporting The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). mandatory medical device reporting: this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. if you are a device user facility, you must. Device Deficiency Reporting.
From www.orielstat.com
Medical Device Incident Reporting Timelines in 6 Major Markets Device Deficiency Reporting the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. the guidance document. Device Deficiency Reporting.
From help.gradelink.com
Deficiency Report Device Deficiency Reporting if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. mandatory medical device reporting: the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). this document addresses good clinical practice for the design, conduct, recording and reporting of. Device Deficiency Reporting.
From present5.com
An Introduction to the Joint Deficiency Reporting System Device Deficiency Reporting the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations.. Device Deficiency Reporting.
From www.youtube.com
How To Create Deficiency Report YouTube Device Deficiency Reporting the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). mandatory medical device reporting: the medical. Device Deficiency Reporting.
From www.sctoplatforms.ch
Safety reporting forms for clinical research projects Tools & Resources Device Deficiency Reporting this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. mandatory medical device reporting: The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. which. Device Deficiency Reporting.
From studylib.net
Clinical Trials Branch Health Sciences Authority Device Deficiency Reporting the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. which reporting forms are to be used for. Device Deficiency Reporting.
From www.scribd.com
Deficiency Report Sample Technology Business Device Deficiency Reporting mandatory medical device reporting: the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. The medical device reporting (mdr) regulation (21. Device Deficiency Reporting.
From www.regdesk.co
FDA Guidance on Medical Device Reporting Specific Issues Device Deficiency Reporting if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. the guidance document safety reporting in clinical investigations. Device Deficiency Reporting.
From www.researchgate.net
Medical Devices Intra ocular devices adverse events reporting and Device Deficiency Reporting the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. mandatory medical device reporting: . Device Deficiency Reporting.
From present5.com
An Introduction to the Joint Deficiency Reporting System Device Deficiency Reporting this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. mandatory medical device reporting: which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. if you are a device user. Device Deficiency Reporting.
From www.scribd.com
Digital Devices Deficiency PDF Device Deficiency Reporting The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. this document addresses good clinical practice for the design,. Device Deficiency Reporting.
From www.signnow.com
Deficiency Log Complete with ease airSlate SignNow Device Deficiency Reporting the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. mandatory medical device reporting: which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. The medical device reporting (mdr). Device Deficiency Reporting.
From www.greenlight.guru
Medical Device Reporting (MDR) How to Take Advantage of Your Device Deficiency Reporting if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations.. Device Deficiency Reporting.
From present5.com
An Introduction to the Joint Deficiency Reporting System Device Deficiency Reporting mandatory medical device reporting: which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities and importers to establish a. the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). The. Device Deficiency Reporting.
From www.templateroller.com
DD Form 1716 Download Fillable PDF or Fill Online Contract Data Package Device Deficiency Reporting mandatory medical device reporting: the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. if you are a. Device Deficiency Reporting.
From www.signnow.com
Deficiency Slip Complete with ease airSlate SignNow Device Deficiency Reporting the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. if you are a device user facility, you must report deaths and serious injuries that a device has or may have caused or. this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. mandatory medical device. Device Deficiency Reporting.
From hxeghawia.blob.core.windows.net
Medical Device Deficiency at Alan Insley blog Device Deficiency Reporting which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). this document addresses good clinical practice for the design,. Device Deficiency Reporting.
From present5.com
An Introduction to the Joint Deficiency Reporting System Device Deficiency Reporting The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. the medical device reporting (mdr) regulation requires medical device manufacturers, device user facilities. Device Deficiency Reporting.
From www.researchgate.net
Adverse event identification and review process. Download Scientific Device Deficiency Reporting this document addresses good clinical practice for the design, conduct, recording and reporting of clinical investigations. mandatory medical device reporting: which reporting forms are to be used for serious adverse events (sae) and device deficiencies (dd) from 26.05.2021?. the guidance document safety reporting in clinical investigations of medical devices under the regulation (eu). the medical. Device Deficiency Reporting.