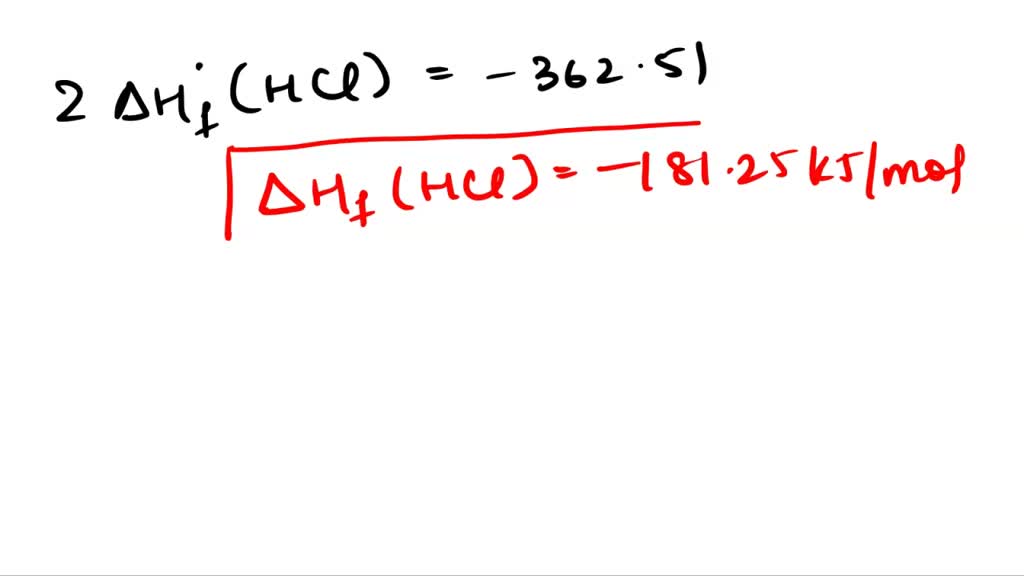Standard Enthalpy Of Formation Of Hcl . in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. The magnitude of δ h for a reaction depends on the physical states of the reactants and the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). standard enthalpies of formation.
from www.numerade.com
The magnitude of δ h for a reaction depends on the physical states of the reactants and the. in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). standard enthalpies of formation. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created.
SOLVED A scientist measures the standard enthalpy change for the
Standard Enthalpy Of Formation Of Hcl in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The magnitude of δ h for a reaction depends on the physical states of the reactants and the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. standard enthalpies of formation. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent.
From www.chegg.com
Solved NH, (g) + HCl(g)ל NH, Cl(s) Given The Standard En... Standard Enthalpy Of Formation Of Hcl the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. The magnitude of δ h for a reaction depends on the physical states of the reactants and the. standard enthalpies of formation. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq,. Standard Enthalpy Of Formation Of Hcl.
From abigailkruwconner.blogspot.com
Enthalpy of Neutralization of Hcl and Naoh Value AbigailkruwConner Standard Enthalpy Of Formation Of Hcl in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. standard enthalpies of formation. The magnitude of δ h for a reaction depends on the physical states of the reactants and the. the. Standard Enthalpy Of Formation Of Hcl.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Enthalpy Of Formation Of Hcl The magnitude of δ h for a reaction depends on the physical states of the reactants and the. standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation. Standard Enthalpy Of Formation Of Hcl.
From www.youtube.com
Calculate the bond enthalpy of HCl, Given that the bond enthalpies of Standard Enthalpy Of Formation Of Hcl the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is. Standard Enthalpy Of Formation Of Hcl.
From www.toppr.com
Bond dissociation enthalpy of H2, Cl2 and HCl are 434, 242 and 431 kJ Standard Enthalpy Of Formation Of Hcl standard enthalpies of formation. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Standard Enthalpy Of Formation Of Hcl.
From www.numerade.com
SOLVED A scientist measures the standard enthalpy change for the Standard Enthalpy Of Formation Of Hcl The magnitude of δ h for a reaction depends on the physical states of the reactants and the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq,. Standard Enthalpy Of Formation Of Hcl.
From www.youtube.com
the standard enthalpy of formation of HCl, H and CL are 92.2 ,217.7 Standard Enthalpy Of Formation Of Hcl the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change. Standard Enthalpy Of Formation Of Hcl.
From www.chegg.com
Solved Using the standard enthalpies of formation listed in Standard Enthalpy Of Formation Of Hcl the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. The magnitude of δ h for a reaction depends on the physical states of the reactants and the. the standard enthalpy. Standard Enthalpy Of Formation Of Hcl.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Of Hcl 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). The magnitude of δ h for a reaction depends on the physical states of the reactants and the. . Standard Enthalpy Of Formation Of Hcl.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Of Hcl in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. standard enthalpies of formation. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a.. Standard Enthalpy Of Formation Of Hcl.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Hydrogen Chloride In Water Equation Standard Enthalpy Of Formation Of Hcl the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). The magnitude of δ h for a reaction depends on the physical states of the reactants and the. the standard. Standard Enthalpy Of Formation Of Hcl.
From www.chegg.com
Solved Enthalpy of Formation HCI + NaOH → NaCl+ H2O HCl Standard Enthalpy Of Formation Of Hcl standard enthalpies of formation. in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200. Standard Enthalpy Of Formation Of Hcl.
From www.slideshare.net
5 3 and 5 4 Standard Enthalpy Of Formation Of Hcl the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change. Standard Enthalpy Of Formation Of Hcl.
From rayb78.github.io
Heat Of Formation Chart Standard Enthalpy Of Formation Of Hcl the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). standard enthalpies of formation. in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its. Standard Enthalpy Of Formation Of Hcl.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Of Hcl in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. standard enthalpies of formation.. Standard Enthalpy Of Formation Of Hcl.
From www.numerade.com
SOLVED Calculate the standard enthalpy change for the reaction at 25Â Standard Enthalpy Of Formation Of Hcl 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. The magnitude of δ h for a reaction depends on the physical states of the reactants and the. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). . Standard Enthalpy Of Formation Of Hcl.
From www.researchgate.net
Solution enthalpy of (CaHap) in the hydrochloric acid solution [HCl Standard Enthalpy Of Formation Of Hcl The magnitude of δ h for a reaction depends on the physical states of the reactants and the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). . Standard Enthalpy Of Formation Of Hcl.
From www.youtube.com
Enthalpy of formation off HF and HCl are 161 kJ and 92 kJ Standard Enthalpy Of Formation Of Hcl The magnitude of δ h for a reaction depends on the physical states of the reactants and the. in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. standard enthalpies of formation. 136. Standard Enthalpy Of Formation Of Hcl.
From www.chegg.com
Solved Use a standard enthalpies of formation table to Standard Enthalpy Of Formation Of Hcl standard enthalpies of formation. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent.. Standard Enthalpy Of Formation Of Hcl.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Of Hcl in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The magnitude. Standard Enthalpy Of Formation Of Hcl.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Of Hcl the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Standard Enthalpy Of Formation Of Hcl.
From www.numerade.com
SOLVEDIf the enthalpy of formation and enthalpy of solution of HCl(g Standard Enthalpy Of Formation Of Hcl the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. The magnitude of δ h for a reaction depends on the physical states of the reactants and the. . Standard Enthalpy Of Formation Of Hcl.
From www.toppr.com
DUNU dissociation enthalpy of ,,C1, and ricare 434, 242 and 431 kJmol Standard Enthalpy Of Formation Of Hcl standard enthalpies of formation. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation. Standard Enthalpy Of Formation Of Hcl.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Of Hcl the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The magnitude of δ h for a reaction depends on the physical states of the reactants. Standard Enthalpy Of Formation Of Hcl.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Of Hcl 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. The magnitude of δ h for a reaction depends on the physical states of the reactants and the. in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard Enthalpy Of Formation Of Hcl.
From byjus.com
Given that bond energies of H H and Cl Cl are 430 kJ /mol and 240 KJ Standard Enthalpy Of Formation Of Hcl in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). the standard enthalpy. Standard Enthalpy Of Formation Of Hcl.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Of Hcl the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. The magnitude of δ h for a. Standard Enthalpy Of Formation Of Hcl.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of Cl(aq) given Standard Enthalpy Of Formation Of Hcl the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. The magnitude of δ h for a reaction depends on the physical states of the reactants and the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. . Standard Enthalpy Of Formation Of Hcl.
From www.bartleby.com
Answered During a neutralization reaction, 1 L… bartleby Standard Enthalpy Of Formation Of Hcl in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. standard enthalpies of formation.. Standard Enthalpy Of Formation Of Hcl.
From byjus.com
Calculate the enthalpy of formation of anhydrous Al2Cl6 from the Standard Enthalpy Of Formation Of Hcl the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The magnitude of δ h for a reaction depends on the physical states of the reactants and the. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq,. Standard Enthalpy Of Formation Of Hcl.
From www.coursehero.com
[Solved] Use standard enthalpies of formation to calculate the enthalpy Standard Enthalpy Of Formation Of Hcl standard enthalpies of formation. the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change. Standard Enthalpy Of Formation Of Hcl.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Of Hcl the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. standard enthalpies of. Standard Enthalpy Of Formation Of Hcl.
From byjus.com
Bond dissociaton enthalpy of H2, Cl2 and HCl are 434, 242 and 431 kJ Standard Enthalpy Of Formation Of Hcl The magnitude of δ h for a reaction depends on the physical states of the reactants and the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of. the standard enthalpy. Standard Enthalpy Of Formation Of Hcl.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Of Hcl the 12 contributors listed below account for 90.3% of the provenance of δ f h° of hcl (aq, 200 h2o). in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. the standard enthalpy. Standard Enthalpy Of Formation Of Hcl.
From www.numerade.com
SOLVEDHow does the bond energy of HCl(g) differ from the standard Standard Enthalpy Of Formation Of Hcl the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent. the. Standard Enthalpy Of Formation Of Hcl.