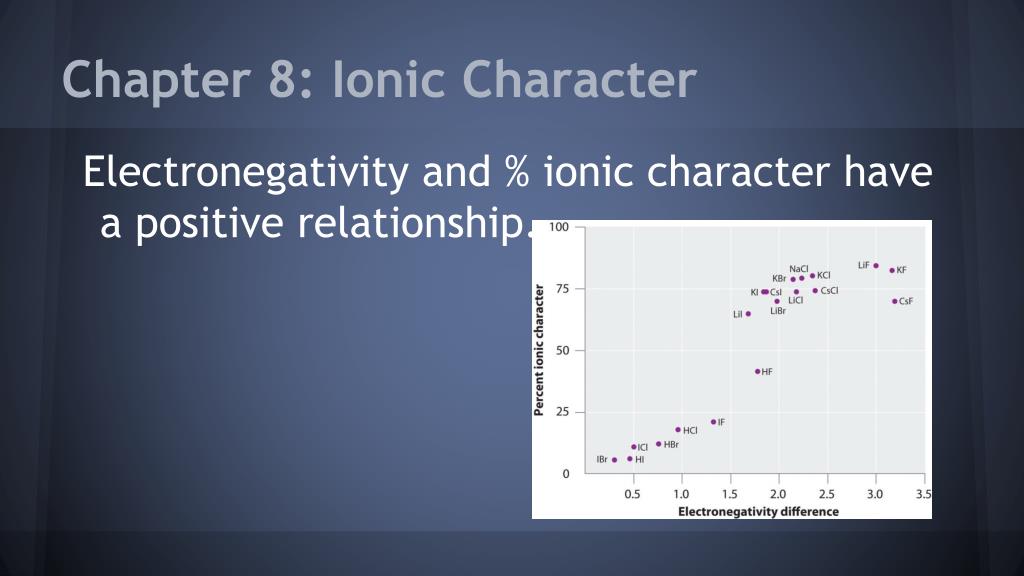
What Is An Ionic Character . a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. what are ionic compounds? This exchange results in a more stable,. It determines how the shared electrons are. learn what ionic character is. the covalent bond is said to have ‘ionic character’. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. A polar covalent bond is. Covalent bonds, and discover how to predict. In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. Trace ionic character trend on the periodic table, examine ionic vs. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in.
from www.slideserve.com
It determines how the shared electrons are. learn what ionic character is. the covalent bond is said to have ‘ionic character’. Covalent bonds, and discover how to predict. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. what are ionic compounds? This exchange results in a more stable,. A polar covalent bond is. a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in.
PPT Chapters 8 & 9 Review PowerPoint Presentation, free download ID
What Is An Ionic Character the covalent bond is said to have ‘ionic character’. Trace ionic character trend on the periodic table, examine ionic vs. learn what ionic character is. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. what are ionic compounds? This exchange results in a more stable,. A polar covalent bond is. In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. It determines how the shared electrons are. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. the covalent bond is said to have ‘ionic character’. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. Covalent bonds, and discover how to predict.
From www.slideserve.com
PPT PowerPoint Presentation ID2453814 What Is An Ionic Character learn what ionic character is. the covalent bond is said to have ‘ionic character’. a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. one way of estimating. What Is An Ionic Character.
From www.numerade.com
SOLVED Ionic Character of Bonds Using the table below, estimate the What Is An Ionic Character This exchange results in a more stable,. It determines how the shared electrons are. Trace ionic character trend on the periodic table, examine ionic vs. what are ionic compounds? In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. electronegativity is a measure of the tendency of an atom to attract. What Is An Ionic Character.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID4493576 What Is An Ionic Character learn what ionic character is. a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. Trace ionic character trend on the periodic table, examine ionic vs. electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. what are ionic. What Is An Ionic Character.
From www.numerade.com
SOLVED Using the figure below, calculate the percent ionic character What Is An Ionic Character Trace ionic character trend on the periodic table, examine ionic vs. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by. What Is An Ionic Character.
From www.chemistrystudent.com
Covalent and Ionic Character (ALevel) ChemistryStudent What Is An Ionic Character A polar covalent bond is. This exchange results in a more stable,. a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. It determines how the shared electrons are. Trace ionic character trend. What Is An Ionic Character.
From sciencenotes.org
Ionic Compound Properties What Is An Ionic Character electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. learn what ionic character is. a nonpolar covalent bond is one. What Is An Ionic Character.
From slideplayer.com
INCREASING IONIC CHARACTER ppt download What Is An Ionic Character one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. the covalent bond is said to have ‘ionic character’. electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. ionic bonding. What Is An Ionic Character.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID4430146 What Is An Ionic Character ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. Covalent bonds, and discover how to predict. a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. A polar covalent bond is. Trace ionic character trend on the. What Is An Ionic Character.
From www.slideserve.com
PPT Chapter 10 Chemical Bonding I Basic Concepts PowerPoint What Is An Ionic Character In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. what are ionic compounds? Trace ionic character trend on the periodic table, examine ionic vs. A polar covalent bond is. one way. What Is An Ionic Character.
From www.slideserve.com
PPT Bonding PowerPoint Presentation, free download ID2858425 What Is An Ionic Character one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. This exchange results in a more stable,. electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. a nonpolar covalent bond is. What Is An Ionic Character.
From www.w3schools.blog
Covalent character of ionic bond W3schools What Is An Ionic Character This exchange results in a more stable,. learn what ionic character is. A polar covalent bond is. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. It determines how the shared electrons are. one way of estimating the ionic. What Is An Ionic Character.
From wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry What Is An Ionic Character A polar covalent bond is. This exchange results in a more stable,. a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. Covalent bonds, and discover how to predict. In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. the covalent bond. What Is An Ionic Character.
From www.youtube.com
percent ionic character worked example problem YouTube What Is An Ionic Character one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. It determines how the shared electrons are. electronegativity is a measure of the tendency. What Is An Ionic Character.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I PART 2 PowerPoint Presentation What Is An Ionic Character It determines how the shared electrons are. the covalent bond is said to have ‘ionic character’. a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. This exchange results in a more stable,. what are ionic compounds? one way of estimating the ionic character of a bond—that. What Is An Ionic Character.
From byjus.com
Determine percentage ionic character of HCl by Henry and Smith formula. What Is An Ionic Character Covalent bonds, and discover how to predict. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. This exchange results in a more stable,. a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. what are ionic. What Is An Ionic Character.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID4430146 What Is An Ionic Character This exchange results in a more stable,. A polar covalent bond is. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. one way of estimating the ionic character of. What Is An Ionic Character.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID3198379 What Is An Ionic Character one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. A polar covalent bond is. Trace ionic character trend on the periodic table, examine ionic vs. what are ionic compounds? learn what ionic character is. This exchange results in a. What Is An Ionic Character.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination What Is An Ionic Character This exchange results in a more stable,. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. A polar covalent. What Is An Ionic Character.
From www.slideserve.com
PPT FTCE Chemistry SAE Preparation Course PowerPoint Presentation What Is An Ionic Character This exchange results in a more stable,. It determines how the shared electrons are. a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. ionic bonding is a type of chemical bond. What Is An Ionic Character.
From www.chegg.com
Solved The percent ionic character of a bond can be approximated What Is An Ionic Character learn what ionic character is. electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. Covalent bonds, and discover how to predict. one way of estimating the. What Is An Ionic Character.
From www.chegg.com
Solved What is the ionic character in the following What Is An Ionic Character the covalent bond is said to have ‘ionic character’. This exchange results in a more stable,. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. ionic bonding is a type of chemical bond in which valence electrons are lost. What Is An Ionic Character.
From www.slideserve.com
PPT Chapters 8 & 9 Review PowerPoint Presentation, free download ID What Is An Ionic Character electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. Covalent bonds, and discover how to predict. This exchange results in a more stable,. Trace ionic character trend on the periodic. What Is An Ionic Character.
From studymind.co.uk
Ionic Formulae & Diagrams (GCSE Chemistry) Study Mind What Is An Ionic Character A polar covalent bond is. This exchange results in a more stable,. Covalent bonds, and discover how to predict. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. a. What Is An Ionic Character.
From solvedlib.com
Using the figure below, calculate the percent ionic c… SolvedLib What Is An Ionic Character the covalent bond is said to have ‘ionic character’. learn what ionic character is. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. Covalent bonds, and discover how to predict. This exchange results in a more stable,. what are ionic compounds? In perfect ionic. What Is An Ionic Character.
From lavelle.chem.ucla.edu
Which has more ionic character? CHEMISTRY COMMUNITY What Is An Ionic Character learn what ionic character is. Covalent bonds, and discover how to predict. Trace ionic character trend on the periodic table, examine ionic vs. the covalent bond is said to have ‘ionic character’. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. one way of. What Is An Ionic Character.
From www.animalia-life.club
Ionic Compounds Periodic Table What Is An Ionic Character what are ionic compounds? a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. A polar covalent bond is. the covalent bond is said to have ‘ionic character’. In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. It determines how. What Is An Ionic Character.
From www.youtube.com
Ionic and Covalent Character in Compounds YouTube What Is An Ionic Character Covalent bonds, and discover how to predict. electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. This exchange results in a more stable,. the covalent bond is said to. What Is An Ionic Character.
From www.numerade.com
SOLVED Using the figure below, calculate the percent ionic character What Is An Ionic Character This exchange results in a more stable,. the covalent bond is said to have ‘ionic character’. learn what ionic character is. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. Covalent bonds, and discover how to predict. what. What Is An Ionic Character.
From www.pathwaystochemistry.com
Naming Simple Ionic Compounds Pathways to Chemistry What Is An Ionic Character what are ionic compounds? learn what ionic character is. electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. This exchange results in a more stable,. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is. What Is An Ionic Character.
From www.slideserve.com
PPT PowerPoint Presentation, free download ID2453814 What Is An Ionic Character the covalent bond is said to have ‘ionic character’. In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. Covalent bonds, and discover how to predict. It determines how the shared electrons are.. What Is An Ionic Character.
From www.sliderbase.com
Ionic Compound Nomenclature Presentation Chemistry What Is An Ionic Character one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. This exchange results in a more stable,. In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. Trace ionic character trend on the periodic table,. What Is An Ionic Character.
From www.slideserve.com
PPT Chap. 4 Chemical Bonding PowerPoint Presentation, free download What Is An Ionic Character In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. learn what ionic character is. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. Trace ionic character trend on the periodic table, examine. What Is An Ionic Character.
From www.chemistrystudent.com
Covalent and Ionic Character (ALevel) ChemistryStudent What Is An Ionic Character In perfect ionic bonding, oppositely charged ions are attracted to each other and can be considered as. A polar covalent bond is. a nonpolar covalent bond is one in which the electrons are shared equally or nearly equally between two atoms. It determines how the shared electrons are. learn what ionic character is. what are ionic compounds?. What Is An Ionic Character.
From www.youtube.com
Periodic Trend Ionic Size YouTube What Is An Ionic Character one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. Trace ionic character trend on the periodic table, examine ionic vs. It determines how the shared electrons are. A polar covalent bond is. one way of estimating the ionic character of. What Is An Ionic Character.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID590973 What Is An Ionic Character one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. one way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in. electronegativity is a. What Is An Ionic Character.