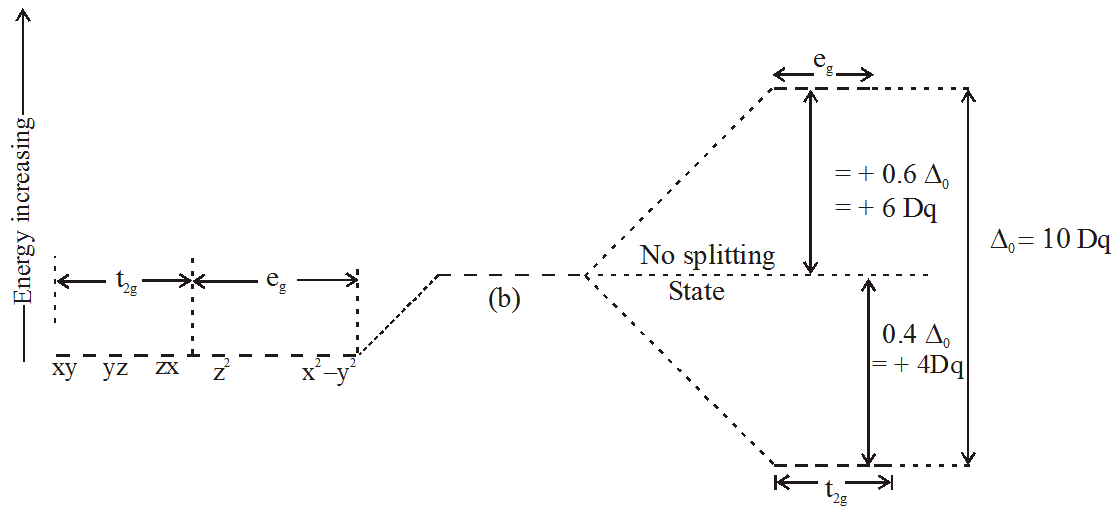
Strong Field Low Spin . The different electron configurations are referred to as high spin (for the weak field case) and low spin (for the strong field case). Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. Ligands that produce a large crystal field splitting, which. From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. The possibility of high and low spin complexes exists for. The higher the oxidation state, the stronger the ligand field, and the more likely the complex will be low spin. Ligand strength can be determined using. The opposite applies to the low spin complexes in which strong field ligands cause. High spin complexes are expected with weak field ligands whereas the crystal field splitting energy is small δ. Low spin complexes with strong field ligands absorb light at shorter wavelengths (higher energy) and high spin complexes with weak field ligands absorb. Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and 5d metal complexes are always low spin.
from www.vedantu.com
From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. Ligands that produce a large crystal field splitting, which. The possibility of high and low spin complexes exists for. Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and 5d metal complexes are always low spin. High spin complexes are expected with weak field ligands whereas the crystal field splitting energy is small δ. The different electron configurations are referred to as high spin (for the weak field case) and low spin (for the strong field case). The higher the oxidation state, the stronger the ligand field, and the more likely the complex will be low spin. Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. Ligand strength can be determined using. Low spin complexes with strong field ligands absorb light at shorter wavelengths (higher energy) and high spin complexes with weak field ligands absorb.
Strong field ligands,such as \\mathrm{CN}^{} usually produce low
Strong Field Low Spin The opposite applies to the low spin complexes in which strong field ligands cause. Ligands that produce a large crystal field splitting, which. The different electron configurations are referred to as high spin (for the weak field case) and low spin (for the strong field case). The opposite applies to the low spin complexes in which strong field ligands cause. From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. The higher the oxidation state, the stronger the ligand field, and the more likely the complex will be low spin. High spin complexes are expected with weak field ligands whereas the crystal field splitting energy is small δ. The possibility of high and low spin complexes exists for. Ligand strength can be determined using. Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and 5d metal complexes are always low spin. Low spin complexes with strong field ligands absorb light at shorter wavelengths (higher energy) and high spin complexes with weak field ligands absorb.
From www.slideserve.com
PPT What Should a Bonding Theory Explain? PowerPoint Presentation Strong Field Low Spin The possibility of high and low spin complexes exists for. From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. The opposite applies to the low spin complexes in which strong field ligands cause. The different electron configurations are referred to as high spin (for. Strong Field Low Spin.
From www.youtube.com
strong field and weak field ligands Low and High spin complex Strong Field Low Spin Ligand strength can be determined using. Low spin complexes with strong field ligands absorb light at shorter wavelengths (higher energy) and high spin complexes with weak field ligands absorb. High spin complexes are expected with weak field ligands whereas the crystal field splitting energy is small δ. The possibility of high and low spin complexes exists for. The opposite applies. Strong Field Low Spin.
From revistas.unam.mx
volumen 33 número 1 Strong Field Low Spin The possibility of high and low spin complexes exists for. The opposite applies to the low spin complexes in which strong field ligands cause. The different electron configurations are referred to as high spin (for the weak field case) and low spin (for the strong field case). Due to effect #2, octahedral 3d metal complexes can be low spin or. Strong Field Low Spin.
From slideplayer.com
Transition Metals and Coordination Chemistry ppt download Strong Field Low Spin From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. The possibility of high and low spin complexes exists for. The opposite applies to the low spin complexes in which strong field ligands cause. Strong field ligands are ones that produce large splittings between the. Strong Field Low Spin.
From www.slideserve.com
PPT Crystal Field Theory PowerPoint Presentation, free download ID Strong Field Low Spin The possibility of high and low spin complexes exists for. Ligand strength can be determined using. Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. Ligands that produce a large crystal field splitting, which. High spin complexes are expected with weak field ligands whereas the crystal field splitting energy is small. Strong Field Low Spin.
From www.vedantu.com
Strong field ligands,such as \\mathrm{CN}^{} usually produce low Strong Field Low Spin High spin complexes are expected with weak field ligands whereas the crystal field splitting energy is small δ. Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. The higher the oxidation state, the stronger the ligand field, and the more likely the complex will be low spin. The different electron configurations. Strong Field Low Spin.
From chemguru.sg
High Spin and Low Spin Complexes Strong Field Low Spin Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and 5d metal complexes are always low spin. Ligand strength can be determined using. High spin complexes are expected with weak field ligands whereas the crystal field splitting energy is small δ. Ligands that produce a large crystal field splitting, which. The possibility. Strong Field Low Spin.
From www.slideserve.com
PPT d 1 PowerPoint Presentation, free download ID828489 Strong Field Low Spin Ligand strength can be determined using. The different electron configurations are referred to as high spin (for the weak field case) and low spin (for the strong field case). From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. The possibility of high and low. Strong Field Low Spin.
From www.youtube.com
Distribution of low spin complexes Iow spin complexes configuration Strong Field Low Spin Ligand strength can be determined using. Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. Low spin complexes with strong field ligands absorb light at shorter wavelengths (higher energy) and high spin complexes with weak field ligands absorb. Ligands that produce a large crystal field splitting, which. The opposite applies to. Strong Field Low Spin.
From chemguru.sg
High Spin and Low Spin Complexes Strong Field Low Spin The possibility of high and low spin complexes exists for. The different electron configurations are referred to as high spin (for the weak field case) and low spin (for the strong field case). Ligand strength can be determined using. Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. The higher the. Strong Field Low Spin.
From www.slideshare.net
Crystal field theory Strong Field Low Spin The opposite applies to the low spin complexes in which strong field ligands cause. The possibility of high and low spin complexes exists for. From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. The higher the oxidation state, the stronger the ligand field, and. Strong Field Low Spin.
From chem.libretexts.org
High Spin and Low Spin Complexes Chemistry LibreTexts Strong Field Low Spin Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. The different electron configurations are referred to as high spin (for the weak field case) and low spin (for the strong field case). Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and 5d. Strong Field Low Spin.
From slideplayer.com
Dear Students of Chemistry 2, ppt download Strong Field Low Spin The different electron configurations are referred to as high spin (for the weak field case) and low spin (for the strong field case). From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. The opposite applies to the low spin complexes in which strong field. Strong Field Low Spin.
From www.researchgate.net
Schematic diagram of the ligand field splitting of the highspin and Strong Field Low Spin Low spin complexes with strong field ligands absorb light at shorter wavelengths (higher energy) and high spin complexes with weak field ligands absorb. The higher the oxidation state, the stronger the ligand field, and the more likely the complex will be low spin. Ligands that produce a large crystal field splitting, which. The different electron configurations are referred to as. Strong Field Low Spin.
From www.slideserve.com
PPT Crystal Field Theory PowerPoint Presentation, free download ID Strong Field Low Spin Ligands that produce a large crystal field splitting, which. The possibility of high and low spin complexes exists for. The higher the oxidation state, the stronger the ligand field, and the more likely the complex will be low spin. The different electron configurations are referred to as high spin (for the weak field case) and low spin (for the strong. Strong Field Low Spin.
From www.slideserve.com
PPT d 1 PowerPoint Presentation, free download ID828489 Strong Field Low Spin From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. Ligands that produce a large crystal field splitting, which. Low spin complexes with strong field ligands absorb. Strong Field Low Spin.
From www.slideserve.com
PPT Crystal Field Theory PowerPoint Presentation, free download ID Strong Field Low Spin High spin complexes are expected with weak field ligands whereas the crystal field splitting energy is small δ. The opposite applies to the low spin complexes in which strong field ligands cause. The possibility of high and low spin complexes exists for. Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and. Strong Field Low Spin.
From ultrafast.stanford.edu
Strongfield Physics Stanford PULSE Institute Strong Field Low Spin High spin complexes are expected with weak field ligands whereas the crystal field splitting energy is small δ. The higher the oxidation state, the stronger the ligand field, and the more likely the complex will be low spin. Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and 5d metal complexes are. Strong Field Low Spin.
From www.slideserve.com
PPT Coordination Chemistry Bonding in transitionmetal complexes Strong Field Low Spin Ligands that produce a large crystal field splitting, which. From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and 5d metal complexes are always low spin. The. Strong Field Low Spin.
From www.slideserve.com
PPT Ligand Field Theory PowerPoint Presentation ID787121 Strong Field Low Spin Ligand strength can be determined using. From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. The opposite applies to the low spin complexes in which strong. Strong Field Low Spin.
From www.slideserve.com
PPT Bonding in complexes of dblock metal ions Crystal Field Theory Strong Field Low Spin From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. The different electron configurations are referred to as high spin (for the weak field case) and low spin (for the strong field case). Low spin complexes with strong field ligands absorb light at shorter wavelengths. Strong Field Low Spin.
From www.slideserve.com
PPT Coordination Chemistry II Ligand Field Theory PowerPoint Strong Field Low Spin The opposite applies to the low spin complexes in which strong field ligands cause. Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and 5d metal complexes are always low spin. From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and. Strong Field Low Spin.
From www.vedantu.com
Strong field ligands,such as \\mathrm{CN}^{} usually produce low Strong Field Low Spin The opposite applies to the low spin complexes in which strong field ligands cause. The higher the oxidation state, the stronger the ligand field, and the more likely the complex will be low spin. Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and 5d metal complexes are always low spin. The. Strong Field Low Spin.
From www.researchgate.net
4 Highand lowenergy spin states in an external field Strong Field Low Spin From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. Ligand strength can be determined using. The possibility of high and low spin complexes exists for. High spin complexes are expected with weak field ligands whereas the crystal field splitting energy is small δ. Due. Strong Field Low Spin.
From mattermodeling.stackexchange.com
transition metals How many spin states do Cu+ and Cu2+ have and why Strong Field Low Spin The possibility of high and low spin complexes exists for. Low spin complexes with strong field ligands absorb light at shorter wavelengths (higher energy) and high spin complexes with weak field ligands absorb. From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. Due to. Strong Field Low Spin.
From www.vedantu.com
Low spin complex of d5cation in an octahedral field will have the Strong Field Low Spin From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. Ligands that produce a large crystal field splitting, which. The higher the oxidation state, the stronger the ligand field, and the more likely the complex will be low spin. Strong field ligands are ones that. Strong Field Low Spin.
From slideplayer.com
Hans Bethe ppt download Strong Field Low Spin From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d. Strong Field Low Spin.
From slideplayer.com
Transition Metals and Coordination Compounds ppt download Strong Field Low Spin From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. Low spin complexes with strong field ligands absorb light at shorter wavelengths (higher energy) and high spin complexes with weak field ligands absorb. Ligand strength can be determined using. The opposite applies to the low. Strong Field Low Spin.
From chem.libretexts.org
High Spin and Low Spin Complexes Chemistry LibreTexts Strong Field Low Spin Ligands that produce a large crystal field splitting, which. The different electron configurations are referred to as high spin (for the weak field case) and low spin (for the strong field case). Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. High spin complexes are expected with weak field ligands whereas. Strong Field Low Spin.
From www.slideserve.com
PPT Coordination Chemistry II Ligand Field Theory PowerPoint Strong Field Low Spin The possibility of high and low spin complexes exists for. Ligand strength can be determined using. The higher the oxidation state, the stronger the ligand field, and the more likely the complex will be low spin. Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and 5d metal complexes are always low. Strong Field Low Spin.
From www.slideserve.com
PPT Chapter 19 PowerPoint Presentation, free download ID5596993 Strong Field Low Spin The opposite applies to the low spin complexes in which strong field ligands cause. Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and 5d metal complexes are always low spin. From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and. Strong Field Low Spin.
From www.slideserve.com
PPT Coordination Chemistry II Ligand Field Theory PowerPoint Strong Field Low Spin Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. The possibility of high and low spin complexes exists for. Ligands that produce a large crystal field splitting, which. Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and 5d metal complexes are always. Strong Field Low Spin.
From www.slideserve.com
PPT Crystal Field Theory (CFT) PowerPoint Presentation, free download Strong Field Low Spin The possibility of high and low spin complexes exists for. Ligand strength can be determined using. Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. Low spin complexes with strong field ligands absorb light at shorter wavelengths (higher energy) and high spin complexes with weak field ligands absorb. Ligands that produce. Strong Field Low Spin.
From shaunmwilliams.com
Chapter 19 Presentation Strong Field Low Spin Strong field ligands are ones that produce large splittings between the d orbitals and form low spin complexes. The possibility of high and low spin complexes exists for. The higher the oxidation state, the stronger the ligand field, and the more likely the complex will be low spin. The opposite applies to the low spin complexes in which strong field. Strong Field Low Spin.
From www.slideserve.com
PPT Crystal Field Theory PowerPoint Presentation, free download ID Strong Field Low Spin From a ligand field theory perspective all three of these ligands have strong \(\pi\) bonds (either double or triple bonds) and have empty \(\pi\)* molecular. Ligand strength can be determined using. The opposite applies to the low spin complexes in which strong field ligands cause. Strong field ligands are ones that produce large splittings between the d orbitals and form. Strong Field Low Spin.